J Breast Cancer.
2015 Dec;18(4):313-322. 10.4048/jbc.2015.18.4.313.
The Association between Dairy Intake and Breast Cancer in Western and Asian Populations: A Systematic Review and Meta-Analysis
- Affiliations
-
- 1Department of Nutrition Hygiene, Shanghai Municipal Center for Disease Control and Prevention, Shanghai, China. zangjiajie0409@163.com
- 2Department of Emergency, Shanghai Armed Policed General Troops Hospital, Shanghai, China.
- 3Department of Nutrition, University of North Carolina at Chapel Hill, Chapel Hill, USA.
- 4Department of Breast & Thyroid Surgery, Affiliated Nanshan Hospital of Guangdong Medical College, Guangzhou, China.
- KMID: 2176279
- DOI: http://doi.org/10.4048/jbc.2015.18.4.313
Abstract
- PURPOSE
To date, studies investigating the association between dairy consumption and breast cancer in women have produced conflicting results. As diet is an important, modifiable factor affecting cancer development, the aim of this study was to examine the association between dairy consumption and breast cancer risk.
METHODS
PubMed, Embase, and Cochrane Library databases were searched with a priority for prospective cohort studies. Case-control studies were also considered in case of the absence of a cohort study.
RESULTS
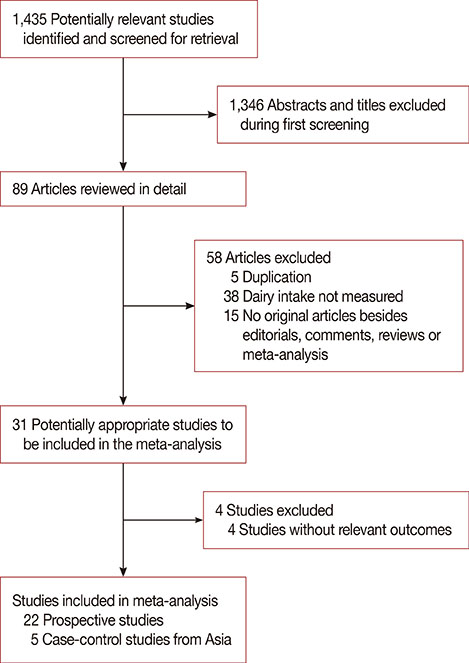
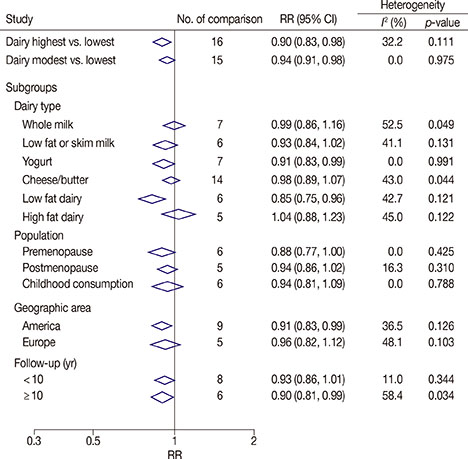
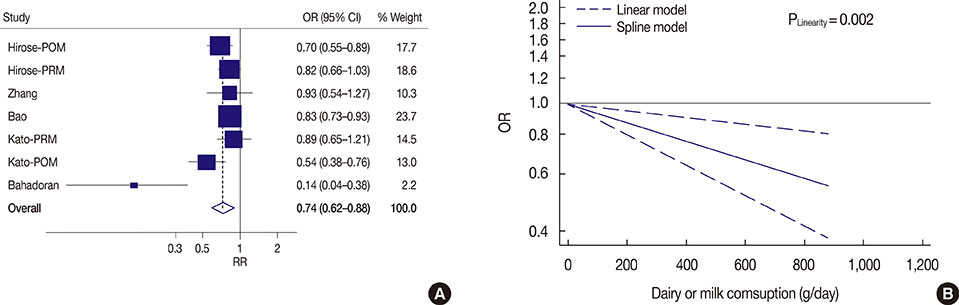
We analyzed 22 prospective cohort studies (1,566,940 participants) and five case-control studies (33,372 participants). High and modest dairy consumption (>600 and 400-600 g/day, respectively) significantly reduced the risk of breast cancer compared with low dairy consumption (<400 g/day; risk ratio [RR], 0.90, 95% confidence interval [CI], 0.83-0.98, and RR, 0.94, 95% CI, 0.91-0.98, respectively). A significant linear relationship between dairy consumption and breast cancer risk was found on dose-response analysis. Subgroup analysis found that yogurt (RR, 0.91; 95% CI, 0.83-0.99) and low-fat dairy (RR, 0.85; 95% CI, 0.75-0.96) reduced the risk of breast cancer, while other dairy product types did not. A reduced risk was observed for people in the United States (RR, 0.91; 95% CI, 0.83-0.99) and in those followed for > or =10 years (RR, 0.90; 95% CI, 0.81-0.99). Additionally, the highest level of dairy consumption among Asians was associated with a reduced risk of breast cancer (odds ratio, 0.74; 95% CI, 0.62-0.88).
CONCLUSION
Dairy consumption was inversely associated with the risk of developing breast cancer and this effect was dependent on the dose, dairy-type, and time.
MeSH Terms
Figure
Reference
-
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.
Article2. Hulka BS, Moorman PG. Breast cancer: hormones and other risk factors. Maturitas. 2008; 61:203–213.
Article3. Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007; 8:292–293.
Article4. Białek A, Tokarz A. Conjugated linoleic acid as a potential protective factor in prevention of breast cancer. Postepy Hig Med Dosw (Online). 2013; 67:6–14.
Article5. Davoodi H, Esmaeili S, Mortazavian AM. Effects of milk and milk products consumption on cancer: a review. Compr Rev Food Sci Food Saf. 2013; 12:249–264.
Article6. Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357:266–281.
Article7. Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of dairy products, calcium, and vitamin D and risk of breast cancer. J Natl Cancer Inst. 2002; 94:1301–1311.
Article8. Probst-Hensch NM, Wang H, Goh VH, Seow A, Lee HP, Yu MC. Determinants of circulating insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations in a cohort of Singapore men and women. Cancer Epidemiol Biomarkers Prev. 2003; 12:739–746.9. Mattisson I, Wirfält E, Johansson U, Gullberg B, Olsson H, Berglund G. Intakes of plant foods, fibre and fat and risk of breast cancer: a prospective study in the Malmö Diet and Cancer cohort. Br J Cancer. 2004; 90:122–127.
Article10. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000; 283:2008–2012.
Article11. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute;Accessed April 20th, 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.12. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006; 6:40–57.
Article13. Crawley H, Mills A, Patel S. Food Standards Agency. Food Portion Sizes. 3rd ed. London: TSO;2002.14. Larsson SC, Orsini N, Wolk A. Dietary magnesium intake and risk of stroke: a meta-analysis of prospective studies. Am J Clin Nutr. 2012; 95:362–366.
Article15. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012; 175:66–73.
Article16. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634.
Article17. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50:1088–1101.
Article18. Xie SP, James SY, Colston KW. Vitamin D derivatives inhibit the mitogenic effects of IGF-I on MCF-7 human breast cancer cells. J Endocrinol. 1997; 154:495–504.
Article19. Xie SP, Pirianov G, Colston KW. Vitamin D analogues suppress IGF-I signalling and promote apoptosis in breast cancer cells. Eur J Cancer. 1999; 35:1717–1723.
Article20. Redaniel MT, Gardner MP, Martin RM, Jeffreys M. The association of vitamin D supplementation with the risk of cancer in postmenopausal women. Cancer Causes Control. 2014; 25:267–271.
Article21. Cauley JA, Chlebowski RT, Wactawski-Wende J, Robbins JA, Rodabough RJ, Chen Z, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women's Health Initiative. J Womens Health (Larchmt). 2013; 22:915–929.
Article22. Lamas B, Nachat-Kappes R, Goncalves-Mendes N, Mishellany F, Rossary A, Vasson MP, et al. Dietary fat without body weight gain increases in vivo MCF-7 human breast cancer cell growth and decreases natural killer cell cytotoxicity. Mol Carcinog. 2015; 54:58–71.
Article23. Turner LB. A meta-analysis of fat intake, reproduction, and breast cancer risk: an evolutionary perspective. Am J Hum Biol. 2011; 23:601–608.
Article24. Sieri S, Krogh V, Ferrari P, Berrino F, Pala V, Thiébaut AC, et al. Dietary fat and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008; 88:1304–1312.
Article25. Schulz M, Hoffmann K, Weikert C, Nöthlings U, Schulze MB, Boeing H. Identification of a dietary pattern characterized by high-fat food choices associated with increased risk of breast cancer: the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Br J Nutr. 2008; 100:942–946.
Article26. Merenstein DJ, Smith KH, Scriven M, Roberts RF, Sanders ME, Petterson S. The study to investigate the potential benefits of probiotics in yogurt, a patient-oriented, double-blind, cluster-randomised, placebo-controlled, clinical trial. Eur J Clin Nutr. 2010; 64:685–691.
Article27. Kang SH, Kim JU, Imm JY, Oh S, Kim SH. The effects of dairy processes and storage on insulin-like growth factor-I (IGF-I) content in milk and in model IGF-I-fortified dairy products. J Dairy Sci. 2006; 89:402–409.
Article28. Dong JY, Zhang L, He K, Qin LQ. Dairy consumption and risk of breast cancer: a meta-analysis of prospective cohort studies. Breast Cancer Res Treat. 2011; 127:23–31.
Article29. Reeves GK, Pirie K, Green J, Bull D, Beral V. Million Women Study Collaborators. Comparison of the effects of genetic and environmental risk factors on in situ and invasive ductal breast cancer. Int J Cancer. 2012; 131:930–937.
Article30. Yoshimoto N, Nishiyama T, Toyama T, Takahashi S, Shiraki N, Sugiura H, et al. Genetic and environmental predictors, endogenous hormones and growth factors, and risk of estrogen receptor-positive breast cancer in Japanese women. Cancer Sci. 2011; 102:2065–2072.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Citrus Fruit Intake and Breast Cancer Risk: A Quantitative Systematic Review
- History of Diabetes Mellitus and Risk of Breast Cancer in Asian Women: A Meta-Epidemiological Analysis of Population-Based Cohort Studies
- The Effects of Depression Intervention Programs for Breast Cancer Patients in Korea : A Systematic Review and Meta-Analysis
- Systematic review and meta-analysis of cancer risks in relation to environmental waste incinerator emissions: a meta-analysis of case-control and cohort studies
- Dietary intake and cancer incidence in Korean adults: a systematic review and meta-analysis of observational studies