J Korean Acad Conserv Dent.
2007 Sep;32(5):459-468. 10.5395/JKACD.2007.32.5.459.
A study of APin-protein interactions using protein microarray
- Affiliations
-
- 1Department of Oral Histology, College of Dentistry, Chosun University, Korea.
- 2Department of Conservative Dentistry, School of Dentistry, Seoul National University, Korea. hhson@snu.ac.kr
- KMID: 2175939
- DOI: http://doi.org/10.5395/JKACD.2007.32.5.459
Abstract
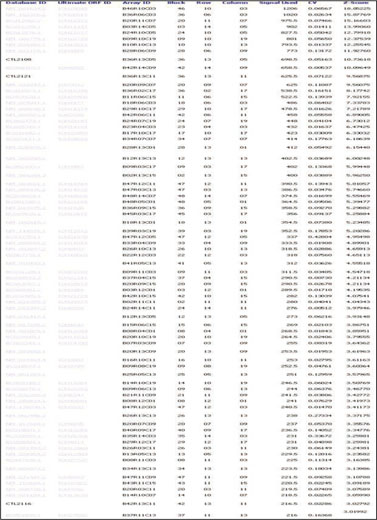
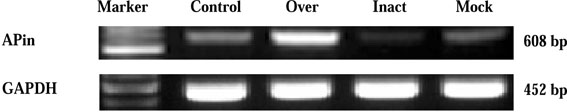
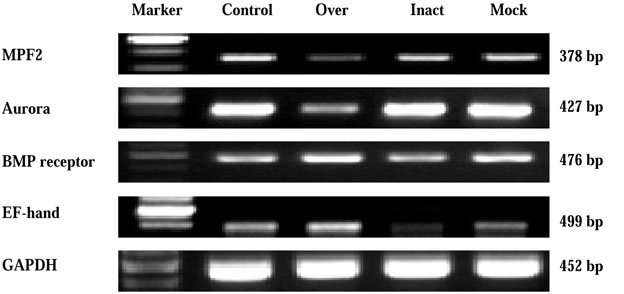
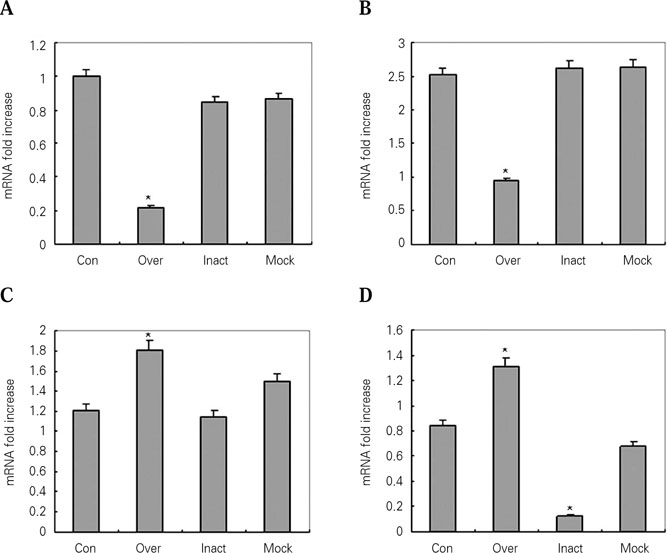
- Protein microarray or protein chips is potentially powerful tools for analysis of protein-protein interactions. APin cDNA was previously identified and cloned from a rat odontoblast cDNA library. The purpose of this study was to investigate the APin-protein interactions during ameloblast differentiation. Protein microarray was carried with recombinant APin protein and MEF2, Aurora kinase A, BMPR-IB and EF-hand calcium binding protein were selected among 74 interacting proteins. Immortalized ameloblast cells (ALCs) were transfected with pCMV-APin construct and U6-APin siRNA construct. After transfection, the expression of the mRNAs for four proteins selected by protein micoarrays were assessed by RT-PCR. The results were as follows: 1. APin expression was increased and decreased markedly after its over-expression and inactivation, respectively. 2. Over-expression of the APin in the ALCs markedly down-regulated the expression of MEF2 and Aurora kinase A, whereas their expression remained unchanged by its inactivation. 3. Expression of BMPR-IB and EF-hand calcium binding protein were markedly increased by the overexpression of the APin in the ALCs, whereas expression of BMPR-IB remained unchanged and expression of EF-hand calcium binding protein was markedly decreased by its inactivation. These results suggest that APin plays an important role in ameloblast differentiation and mineralization by regulating the expression of MEF2, Aurora kinase A, BMPR-IB and EF-hand calcium binding protein.
Keyword
MeSH Terms
Figure
Reference
-
1. Mina M, Kollar EJ. The induction of odontogenesis in non-dental mesinchyme combined with early murine mandibular arch epithelium. Arch oral Biol. 1987. 32:123–127.
Article2. D'Souza RN, Cavender A, Sunavala G, Alvarez J, Ohshima T, Kulkarni AB, MacDougall M. Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J Bone Miner Res. 1997. 12:2040–2049.3. Dey R, Son HH, Cho MI. Isolation and partial sequencing of potentially odontoblast-specific/enriched rat cDNA clones obtained by suppression subtractive hybridiztion. Arch Oral Biol. 2001. 46:249–260.
Article4. Solomon A, Murphy CL, Weaver K, Sletten K, Westermark G, Westermark P. Calcifying epithelial odontogenic (Pindborg) tumor-associated amyloid consists of a novel human protein. J Lab Clin Med. 2003. 142:348–355.
Article5. Kim DH, Kim HJ, Jeong MJ, Son HH, Park JC. Expression and functional characterization of odontoblast-derived gene: OD314. J Korean Acad Conserv Dent. 2004. 29:399–408.
Article6. Park JC, Kim IH, Kim HJ, Jeong MJ, Oh HJ, Jeong JO, Son HH. Role of OD314 During Odontoblast Differentiation. Korean J Phys Anthropol. 2005. 18:187–196.
Article7. Aung PP, Oue N, Mitani Y, Bosserhoff AK, Yasui W. Systematic search for gastric cancer-specific genes based on SAGE data: melanoma inhibitory activity and matrix metalloproteinase-10 are novel prognostic factors in patients with gastric cancer. Oncogene. 2006. 25:2546–2557.
Article8. Moffatt P, Smith CE, Sooknanan R, St-Arnaud R, Nanci A. Identification of secreted and membrane proteins in the rat incisor enamel organ using a signal-trap screening approach. Eur J Oral Sci. 2006. 114:Suppl 1. 139–146.
Article9. Kawahashi Y, Doi N, Takashima H, Tsuda C, Oishi Y, Oyama R, Yonezawa M, Miyamoto-Sato E, Yanagawa H. In vitro protein microarrays for detecting protein-protein interactions: application of a new method for fluorescence labeling of proteins. Proteomics. 2003. 3:1236–1243.
Article10. Letarte M, Voulgaraki D, Hatherley D, Foster-Cuevas M, Saunders NJ, Barclay AN. Analysis of leukocyte membrane protein interactions using protein microarrays. BMC Biochem. 2005. 6:2.11. Hidaka K, Morisaki T, Byun SH, Hashido K, Toyama K, Mukai T. The MEF2B homologue differentially expressed in mouse embryonal carcinoma cells. Biochem Biophys Res Commun. 1995. 213:555–560.
Article12. Yu YT, Breitbart RE, Smoot LB, Lee Y, Mahdavi V, Nadal-Ginard B. Human myocyte-specific enhancer factor 2 comprises a group of tissue-restricted MADS box transcription factors. Genes Dev. 1992. 6:1783–1798.
Article13. Brown JR, Koretke KK, Birkeland ML, Sanseau P, Patrick DR. Evolutionary relationships of Aurora kinases: implications for model organism studies and the development of anti-cancer drugs. BMC Evol Biol. 2004. 4:39–45.14. Carvajal RD, Tse A, Schwartz GK. Aurora kinases: new targets for cancer therapy. Clin Cancer Res. 2006. 12:6869–6875.
Article15. Katayama H, Brinkley WR, Sen S. The Aurora kinases: role in cell transformation and tumorigenesis. Cancer Metastasis Rev. 2003. 22:451–464.16. Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, Lyons KM, Mishina Y, Seykora JT, Crenshaw EB 3rd, Millar SE. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004. 131:2257–2268.
Article17. Nadiri A, Kuchler-Bopp S, Perrin-Schmitt F, Lesot H. Expression patterns of BMPRs in the developing mouse molar. Cell Tissue Res. 2006. 324:33–40.
Article18. Davideau JL, Celio MR, Hotton D, Berdal A. Developmental pattern and subcellular localization of parvalbumin in the rat tooth germ. Arch Oral Biol. 1993. 38:707–715.
Article19. Deutsch D, Dafni L, Palmon A, Hekmati M, Young MF, Fisher LW. Tuftelin: enamel mineralization and amelogenesis imperfecta. Ciba Found Symp. 1997. 205:135–147.
Article20. Sui G, Soohoo C, Affar el B, Gay F, Shi Y, Forrester WC, Shi Y. DNA vector-based RNA: technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci USA. 2002. 99:5515–5520.
Article21. Kim HJ, Jeong MJ, Son HH, Park JC. Inactivation of the OD314 Gene by RNA Interference in Preodontoblast Cell Lines. Korean J Phys Anthropol. 2004. 17:121–129.
Article22. Nakata A, Kameda T, Nagai H, Ikegami K, Duan Y, Terada K, Sugiyama T. Establishment and characterization of a spontaneously immortalized mouse ameloblast-lineage cell line. Biochem Biophys Res Commun. 2003. 308:834–839.
Article23. Kim JW. APin Chaechohap tanbaekchil ui hapsongkwa chogyong. 2006. Chosun University;MD thesis.24. Bei M, Stowell S, Maas R. Msx2 controls ameloblast terminal differentiation. Dev Dyn. 2004. 231:758–765.25. Millar SE, Koyama E, Reddy ST, Andl T, Gaddapara T, Piddington R, Gibson CW. Over- and ectopic expression of Wnt3 causes progressive loss of ameloblasts in postnatal mouse incisor teeth. Connect Tissue Res. 2003. 44:124–129.
Article26. Wang XP, Suomalainen M, Jorgez CJ, Matzuk MM, Werner S, Thesleff I. Follistatin regulates enamel patterning in mouse incisors by asymetrically inhibitting BMP signaling and ameloblast differentiation. Dev Cell. 2004. 7:719–730.
Article27. Garant PR. Oral cells and tissues. 2003. 2nd ed. Chicago, IL: Quintessence publishing Co., Inc.;25–52.28. Nancy A. Ten Cate's oral histology: development, structure, and function. 2003. 6th ed. St. Louis, MI: Mosby, Inc.;192–239.29. Moffatt P, Smith CE, St-Arnaud R, St-Arnaud R, Nanci A. Cloning of rat amelotin and localization of the protein to the basal lamina of maturation stage ameloblasts and junctional epithelium. Biochem J. 2006. 399:37–46.
Article30. Reith EJ, Boyde A. Autoradiographic evidence of cyclical entry of calcium into maturing enamel of the rat incisor tooth. Arch Oral Biol. 1981. 26:983–987.
Article31. Salama AH, Zaki AE, Eisenmann DR. Cytochemical localization of Ca2+ - Mg2+ adenosine triphosphatase in rat incisor ameloblasts during enamel secretion and maturation. J Histochem Cytochem. 1987. 35:471–482.
Article32. Smith CE. Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med. 1998. 9:128–161.
Article33. Takano Y, Crenshaw MA, Reith EJ. Correlation of 45Ca incorporation with maturation ameloblast morphology in the rat incisor. Calcif Tissue Int. 1982. 34:211–213.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Microarray Data Analysis of Perturbed Pathways in Breast Cancer Tissues
- Computational approaches for molecular characterization and structure-based functional elucidation of a hypothetical protein from Mycobacterium tuberculosis
- Small Molecule Inhibitors to Disrupt Protein-protein Interactions of Heat Shock Protein 90 Chaperone Machinery
- Expression and function of OD314, Apin protein during ameloblast differentiation and amelogenesis
- A protein interactions map of multiple organ systems associated with COVID-19 disease