J Korean Acad Conserv Dent.
2007 Sep;32(5):403-410. 10.5395/JKACD.2007.32.5.403.
Tissue response of Pro-Root(R) MTA with rhBMP-2 in pulpotomized rat teeth
- Affiliations
-
- 1Division of Conservative Dentistry, Department of Dentistry, Asan Medical Center, Ulsan University, Seoul, Korea. kmr333@amc.seoul.kr
- KMID: 2175931
- DOI: http://doi.org/10.5395/JKACD.2007.32.5.403
Abstract
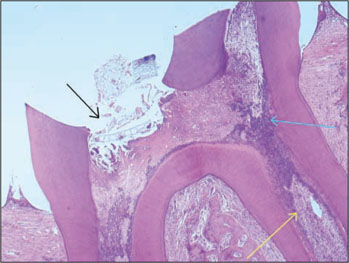
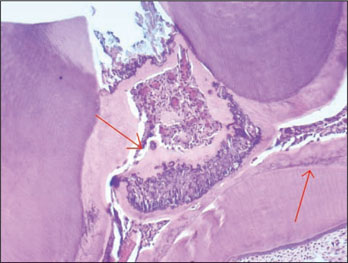
- The purpose of this study was to investigate whether rhBMP-2 (BMP2) could induce synergistic effect with Pro-Root(R) MTA (MTA) in pulpotomized teeth in the rats. Healthy upper first molars from thirty-two, 10 weeks old, Sprague-Dawley rats were used for this investigation. The molars were exposed with round bur, and light pressure was applied with sterilized cotton to control hemorrhage. 1.2 grams of MTA cement was placed in right first molars as a control group. In left first molars, 1 microg of BMP2 was additionally placed on exposed pulps with MTA. All cavities were back-filled with light-cured glass-ionomer cements. The rats were sacrificed after 2 weeks and 7 weeks, respectively. Then histologic sections were made and assessed by light microscopy. Data were statistically analyzed via student t-test with SPSSWIN 12.0 program (p < 0.05). Inflammation observed in 2 weeks groups were severe compared to the 7 weeks groups. But the differences were not statistically significant. BMP2-addition groups had less inflammation than MTA groups in both periods, though these differences were also not statistically significant. In conclusion, the combination of BMP2 and MTA showed no differences with MTA only for pulpotomy of rat teeth.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
A bioactivity study of Portland cement mixed with β-glycerophosphosphate on human pulp cell
Young-Hwan Oh, Young-Joo Jang, Yong-Bum Cho
J Korean Acad Conserv Dent. 2009;34(5):415-423. doi: 10.5395/JKACD.2009.34.5.415.
Reference
-
1. Schroder U. Effects of calcium hydroxide-containing pulp-capping agents on pulp cell migration, proliferation, and differentiation. J Dent Res. 1985. 64:541–548.
Article2. Tziafas D. The future role of a molecular approach to pulp-dentinal regeneration. Caries Res. 2004. 38:314–320.
Article3. Ranly DM, Garcia-Godoy FJ. Current and potential pulp therapies for primary and young permanent teeth. J Dent. 2000. 28:153–161.
Article4. Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999. 25:197–205.
Article5. Peng L, Ye L, Tan H, Zhou X. Evaluation of the formocresol versus mineral trioxide aggregate primary molar pulpotomy: a meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006. 102:e40–e44.
Article6. Abedi HR, Torabinejad M, Pitt Ford TR. Using mineral trioxide aggregate as a pulp-capping material. J Am Dent Assoc. 1996. 127:1491–1494.
Article7. Ford TR, Torabinejad M, Abedi HR, Bakland LK, Kariyawasam SP. Using mineral trioxide aggregate as a pulp-capping material. J Am Dent Assoc. 1996. 127:1491–1494.
Article8. Faraco IM, Holland R. Response of the pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent Traumatol. 2001. 17:163–166.
Article9. Mitchell PJ, Pitt Ford TR, Torabinejad M, McDonald F. Osteoblast biocompatibility of mineral trioxide aggregate. Biomaterials. 1999. 20:167–173.
Article10. Koh ET, McDonald F, Pitt Ford TR, Torabinejad M. Cellular response to Mineral Trioxide Aggregate. J Endod. 1998. 24:543–547.
Article11. Koh ET, Torabinejad M, Pitt Ford TR, Brady K, McDonald F. Mineral trioxide aggregate stimulates a biological response in human osteoblasts. J Biomed Mater Res. 1997. 37:432–439.
Article12. Torabinejad M, Rastegar A, Kattering J, Pitt Ford TR. Bacterial microleakage of mineral trioxide aggregate as a root-end filling material. J Endod. 1995. 21:109–112.
Article13. Al-Nazhan S, Al-Judai A. Evaluation of antifungal activity of mineral trioxide aggregate. J Endod. 2003. 29:826–827.
Article14. Al-Hezaimi K, Al-Shalan TA, Naghshbandi J, Oglesby S, Simon JH, Rotstein I. Antibacterial effect of two mineral trioxide aggregate(MTA) preparations against Enterococcus Faecalis and Streptococcus sanguis in vitro. J Endod. 2006. 32:1053–1056.
Article15. Aeinehchi M, Eslami B, Ghanbariha M, Saffar AS. Mineral trioxide aggregate(MTA) and calcium hydroxide as pulp capping agents in human teeth; a preliminary report. Int Endod J. 2003. 36:225–231.16. Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod. 2005. 31:711–718.
Article17. Sloan AJ, Smith AJ. Stem cells and the dental pulp:potential roles in dentine regeneration and repair. Oral Dis. 2007. 13:151–157.
Article18. Wozney JM. The bone morphogenetic protein family: multifunctional cellular regulators in the embryo and adult. Eur J Oral Sci. 1998. 106:160–166.
Article19. Lianjia Y. Immunohistochemical localization of bone morphogenetic protein(BMP) in calcifying fibrous epulis. J Oral Pathol Med. 1993. 22:406–410.
Article20. Tziafas D, Kolokuris I. Inductive influences of demineralized dentin and bone matrix on pulp cells: an approach of secondary dentinogenesis. J Dent Res. 1990. 69:75–81.
Article21. Smith AJ, Tobias RS, Cassidy N, Begue-Kirn C, Ruch JV, Lesot H. Influence of substrate nature and immobilization of implanted dentin matrix components during induction of reparative dentinogenesis. Connect Tissue Res. 1995. 32:291–296.
Article22. Nakashima M. Induction of dentin formation on canine amputated pulp by recombinant human bone morphogenetic proteins (BMP)-2 and -4. J Dent Res. 1994. 73:1515–1522.
Article23. Canalis E, McCarthy T, Centrella M. Growth factors and the regulation of bone remodelling. J Clin Invest. 1988. 81:277–281.24. Hu CC, Zhang C, Quan Q, Tatum NB. Reparative dentin formation in rat molars after direct pulp capping with growth factors. J Endod. 1998. 24:744–751.
Article25. Kim MR, Kim BH, Yoon SH. Effect on the healing of periapical perforations in dogs of the addition of growth factors to calcium hydroxide. J Endod. 2001. 27:734–737.
Article26. Giannobile WV. Periodontal tissue engineering by growth factors. Bone. 1996. 23S–37S.
Article27. Nakashima M. Induction of dentine in amputated pulp of dogs by recombinant human bone morphogenetic proteins-2 and -4 with collagen matrix. Arch Oral Biol. 1994. 39:1085–1089.
Article28. Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003. 21:1025–1032.
Article29. Bègue-Kirn C, Smith AJ, Loriot M, Kupferle C, Ruch JV, Lesot H. Comparative analysis of TGF-βs, BMPs, IGF, msxs, fibronectin, osteonectin and bone sialoprotein gene gene expression during normal and in vitro induced odontoblast differentiation. Int J Dev Biol. 1994. 38:405–420.30. Torabinejad M, Kutsenko D, Machnick T, Ismail A, Newton CW. Levels of evidence for the outcome of nonsurgical endodontic treatment. J Endod. 2005. 31:637–646.
Article31. Iohara K, Nakashima M, Ito M, Ishikawa M, Nakashima A, Akamine A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res. 2004. 83:590–595.
Article32. Kim MR, Ko HJ, Yang WK, Kim WK, Lee YK. Cytotoxicity of ProRoot® MTA cement with rhBMP-2. J Korean Acad Endod. 2006. 7:39–49.33. Six N, Lasfargues JJ, Goldberg M. Differential repair responses in the coronal and radicular areas of the exposed rat molar pulp induced by recombinant human bone morphogenetic protein 7 (osteogenic protein 1). Arch Oral Biol. 2002. 47:177–187.
Article34. Goldberg M, Six N, Decup F, Lasfargues JJ, Salih E, Tompkins K, Veis A. Bioactive molecules and the future of pulp therapy. Am J Dent. 2003. 16:66–76.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Use of mineral trioxide aggregate in the treatment of horizontal root fracture with a 4-year follow-up: case report
- Spectrophotometric evaluation of sealing effects of several root-end filling materials
- Effects of different calcium-silicate based materials on fracture resistance of immature permanent teeth with replacement root resorption and osteoclastogenesis
- Failure of orthograde MTA filling: MTA wash-out?
- Evaluation of the rat tissue reaction to experimental new resin cement and mineral trioxide aggregate cement