Diabetes Metab J.
2011 Jun;35(3):243-247. 10.4093/dmj.2011.35.3.243.
Angiotensin II Inhibits Insulin Binding to Endothelial Cells
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea. sonhys@gmail.com
- 2Catholic Research Institutes of Medical Science, The Catholic University of Korea, Seoul, Korea.
- KMID: 2175427
- DOI: http://doi.org/10.4093/dmj.2011.35.3.243
Abstract
- BACKGROUND
Insulin-mediated glucose uptake in insulin target tissues is correlated with interstitial insulin concentration, rather than plasma insulin concentration. Therefore, insulin delivery to the interstitium of target tissues is very important, and the endothelium may also play an important role in the development of insulin resistance.
METHODS
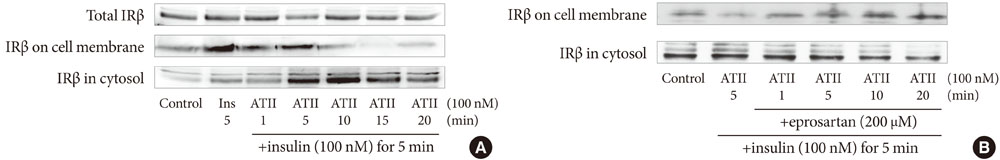
After treating bovine aortic endothelial cells with angiotensin II (ATII), we observed the changes in insulin binding capacity and the amounts of insulin receptor (IR) on the cell membranes and in the cytosol.
RESULTS
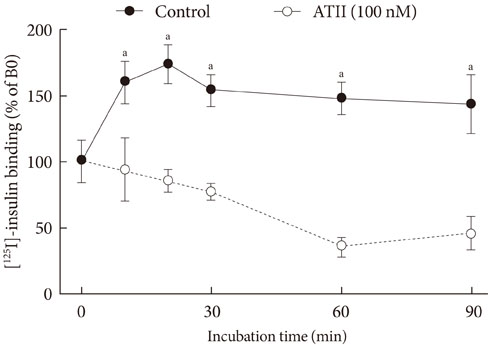
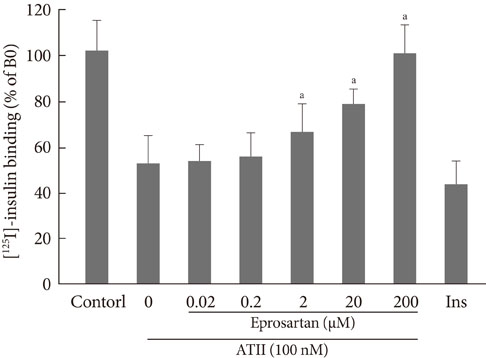
After treatment of 10(-7)M ATII, insulin binding was decreased progressively, up to 60% at 60 minutes (P<0.05). ATII receptor blocker (eprosartan) dose dependently improved the insulin binding capacity which was reduced by ATII (P<0.05). At 200 microM, eprosartan fully restored insulin binding capacity, althogh it resulted in only a 20% to 30% restoration at the therapeutic concentration. ATII did not affect the total amount of IR, but it did reduce the amount of IR on the plasma membrane and increased that in the cytosol.
CONCLUSION
ATII decreased the insulin binding capacity of the tested cells. ATII did not affect the total amount of IR but did decrease the amount of IR on the plasma membrane. Our data indicate that ATII decreases insulin binding by translocating IR from the plasma membrane to the cytosol. The binding of insulin to IR is important for insulin-induced vasodilation and transendothelial insulin transport. Therefore, ATII may cause insulin resistance through this endothelium-based mechanism.
Keyword
MeSH Terms
Figure
Reference
-
1. Yang YJ, Hope ID, Ader M, Bergman RN. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest. 1989. 84:1620–1628.2. Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999. 399:601–605.3. King GL, Johnson SM. Receptor-mediated transport of insulin across endothelial cells. Science. 1985. 227:1583–1586.4. Eggleston EM, Jahn LA, Barrett EJ. Hyperinsulinemia rapidly increases human muscle microvascular perfusion but fails to increase muscle insulin clearance: evidence that a saturable process mediates muscle insulin uptake. Diabetes. 2007. 56:2958–2963.5. Wang H, Liu Z, Li G, Barrett EJ. The vascular endothelial cell mediates insulin transport into skeletal muscle. Am J Physiol Endocrinol Metab. 2006. 291:E323–E332.6. Steil GM, Ader M, Moore DM, Rebrin K, Bergman RN. Transendothelial insulin transport is not saturable in vivo. No evidence for a receptor-mediated process. J Clin Invest. 1996. 97:1497–1503.7. Vicent D, Ilany J, Kondo T, Naruse K, Fisher SJ, Kisanuki YY, Bursell S, Yanagisawa M, King GL, Kahn CR. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest. 2003. 111:1373–1380.8. Kaiser N, Vlodavsky I, Tur-Sinai A, Fuks Z, Cerasi E. Binding, internalization, and degradation of insulin in vascular endothelial cells. Diabetes. 1982. 31:1077–1083.9. Sjostrand M, Holmang A, Lonnroth P. Measurement of interstitial insulin in human muscle. Am J Physiol. 1999. 276(1 Pt 1):E151–E154.10. Sjostrand M, Gudbjornsdottir S, Holmang A, Lonn L, Strindberg L, Lonnroth P. Delayed transcapillary transport of insulin to muscle interstitial fluid in obese subjects. Diabetes. 2002. 51:2742–2748.11. Herkner H, Klein N, Joukhadar C, Lackner E, Langenberger H, Frossard M, Bieglmayer C, Wagner O, Roden M, Muller M. Transcapillary insulin transfer in human skeletal muscle. Eur J Clin Invest. 2003. 33:141–146.12. Wascher TC, Wolkart G, Russell JC, Brunner F. Delayed insulin transport across endothelium in insulin-resistant JCR:LA-cp rats. Diabetes. 2000. 49:803–809.13. Simionescu M, Gafencu A, Antohe F. Transcytosis of plasma macromolecules in endothelial cells: a cell biological survey. Microsc Res Tech. 2002. 57:269–288.14. Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury. Part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003. 108:1912–1916.15. Imanishi T, Kobayashi K, Kuroi A, Mochizuki S, Goto M, Yoshida K, Akasaka T. Effects of angiotensin II on NO bioavailability evaluated using a catheter-type NO sensor. Hypertension. 2006. 48:1058–1065.16. Andreozzi F, Laratta E, Sciacqua A, Perticone F, Sesti G. Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circ Res. 2004. 94:1211–1218.17. Chisalita SI, Arnqvist HJ. Insulin-like growth factor I receptors are more abundant than insulin receptors in human micro- and macrovascular endothelial cells. Am J Physiol Endocrinol Metab. 2004. 286:E896–E901.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Study of effects of complex aerobatics on serum insulin, cortisol and angiotensin II
- The effects of angiotensin II and thrombin on the secretion of PDGF endothelin, and PGI2 in cultured umbilical vein endothelial cells
- Regulation of Angiotensin II Binding in Renal Proximal Tubule Cells by High Glucose : I.Involvement of PKC, Ca2+, and Oxidative Stress
- Epigallocatechin-3-gallate Regulates Inducible Nitric Oxide Synthase Expression in Human Umbilical Vein Endothelial Cells
- Overview of the Renin-Angiotensin System