J Breast Cancer.
2008 Mar;11(1):10-17. 10.4048/jbc.2008.11.1.10.
Restoration of Reproductive Potential after Autotransplantation of Frozen Ovaries in Mice Pretreated with Cyclophosphamide
- Affiliations
-
- 1Department of Surgery and 1Surgical Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea. cjahn1220@hanmir.com
- KMID: 2175042
- DOI: http://doi.org/10.4048/jbc.2008.11.1.10
Abstract
-
PURPOSE: Infertility due to ovarian failure that is caused by antineoplastic chemotherapeutic agents is one of the primary problems of female cancer atients who are in their reproductive years. It has become important to preserve the reproductive potential of female cancer patients. This study was conducted to determine whether autotransplantation of frozen ovaries can restore reproductive potential.
METHODS
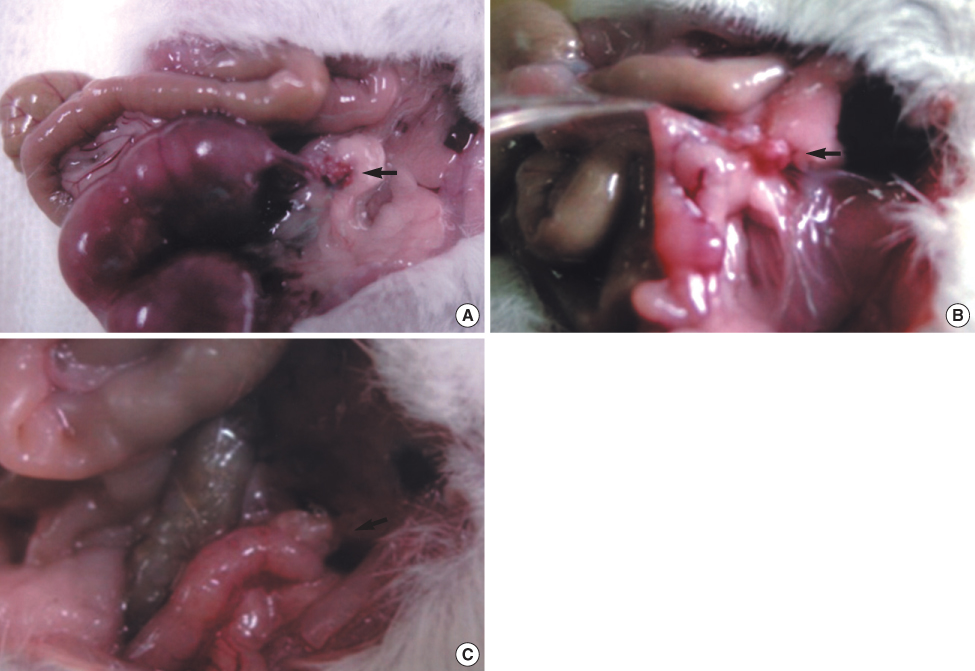
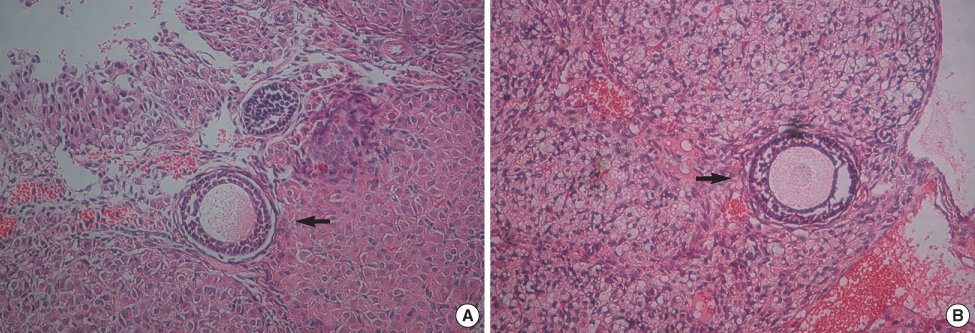
This study included 30 female mice that had normal reproductive potential. The mice were divided into 4 groups: the positive control, the negative control, the comparison group, and the experimental group. The positive control group received right total oophorectomy, and the negative control group received bilateral total oophorectomy. Greater than or equal to 90% of the left ovary was removed in the mice of the comparison group, and then cyclophosphamide was administered. In the experimental group, the right ovary taken out by right total oophorectomy, and this was crypreserved using the vitrification method. And then cyclophosphamide was administered. The cryopreserved ovary was autotransplanted to the left gonadal fat pad after greater than or equal to 90% of the left ovary was removed. The reproductive performance in each group was analyzed according to the pregnancy rate after mating.
RESULTS
In the positive control group, all five mice became pregnant, and the number of fetuses was 4 to 5 (mean=4.60+/-0.55). In the comparison group, the pregnancy rate was 50%, and the mean number of fetuses was 1.40+/-0.55. In the experimental group, 7 of 10 (70%) mice became pregnant, and the mean number of fetuses was 4.71+/-2.56. There was no significant difference in the number of fetuses between the positive control and the experimental group (p=0.093), but there was a significant difference in the number of fetuses between the comparison group and the experimental group (p=0.019).
CONCLUSION
The results of this study suggest that autotransplantation of frozen ovaries using the vitrification method may restore the impaired ovarian function induced by antineoplastic chemotherapeutic agents.
Keyword
MeSH Terms
Figure
Reference
-
1. Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at -196℃. Hum Reprod. 1994; 9:597–603.2. Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. A Live birth after orthotopic transplatation of cryopreserved ovarian tissue. Lancet. 2004; 364:1405–1410.3. Gunasena KT, Villines PM, Critser ES, Critser JK. Live births after autologous transplant of cryopreserved mouse ovaries. Hum Reprod. 1997; 12:101–106.
Article4. Meirow D, Lewin A, Or R, Rachmilewitz E, Slavin S, Schenker JG, et al. Ovarian failure post-chemotherapy in young cancer patients-risk assessment indicate the need for intervention. Fert Steril. 1997; 68 Suppl 1:S218.5. Familiari G, Caggiati A, Nottola SA, Ermini M, Di Benedetto MR, Motta PM. Infertility: ultrastructure of human ovarian primordial follicles after combination chemotherapy for Hodgkin\'s disease. Hum Reprod. 1993; 8:2080–2087.
Article6. Trounson A, Mohr L. Human pregnancy following cryopreservation. thawing and transfer of an eight cell embryo. Nature. 1983; 305:707–709.7. Chen C. Pregnancy after human oocyte cryopreservation. Lancet. 1986; 1:884–886.
Article8. Ataya K, Ramahi-Ataya A. Reproductive performance of female rats treated with cyclophosphamide and/or LHRH agonist. Reprod Toxicol. 1993; 7:229–235.
Article9. Bramley TA, Menzies GS. Measurement of luteal and placental gonadotrophin-releasing hormone (GnRH) binding sites: role of inactivation of GnRH tracer. Mol Hum Reprod. 1996; 2:535–539.10. Park MH, Yoon HC, Yoon JH, Jegal YJ. The efficacy of oral combination chemotherapy of 5\'-DFUR and cyclophosphamide for metastatic breast cancer. J Korean Breast Cancer Society. 2006; 9:249–253.
Article11. Nakao K, Nakagata N, Katsuki M. Simple and efficient vitrification procedure for cryopreservation of mouse embryo. Exp Anim. 1997; 46:231–234.12. Parrot DM. The fertility of mice with orthotopic ovarian grafts derived from frozen tissue. J Reprod Fertil. 1960; 1:230–241.13. Lee DM, Yeoman RR, Battaglia DE, Stouffer RL, Zelinski-Wooten MB, Fanton JW, et al. Live birth after ovarian tissue transplant. Nature. 2004; 428:137–138.
Article14. Oktay K, Karlikaya G, Gosden R, Schwarz R. Ovarian function after autologous transplantation of frozen-banked human ovarian tissue (abstract O-054). Fertil Steril. 1999; 72(3 Suppl 1):S21.15. Meirow D, Lewis H, Nugent D, Epstein M. Subclinical depletion of primordial follicular reserve in mice treated with cyclophosphamide: clinical importance and proposed accurate investigative tool. Hum Reprod. 1999; 14:1903–1907.
Article16. Harp R, Leibach J, Black J, Keldahl C, Karow A. Cryopreservation of murine ovarian tissue. Cryobiology. 1994; 31:336–343.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preservation of ovarian follicle by concomitant administration of GnRH agonist I, II or GnRH antagonist during Cyclophosphamide or Paclitaxel chemotherapy in mice
- Expression of angiogenic factors in cryopreserved mouse ovaries after heterotopic autotransplantation
- Modulatory effect of water and/or food deprivation, and cyclophosphamide administration on immune response in mice
- Restoration of Adriamycin and Vincristine Dependent Tumoricidal Activity by Interferon in Mice with Implanted Tumor Cells
- Ovarian Development of Vitrified Neonatal Ovaries after Orthotopic Transplantation into Adult Recipients