Intest Res.
2014 Jul;12(3):214-220. 10.5217/ir.2014.12.3.214.
The Effect of Infliximab on Patients with Ulcerative Colitis in Korea
- Affiliations
-
- 1Department of Internal Medicine, Ulsan University College of Medicine, Gangneung Asan Hospital, Gangneung, Korea.
- 2Department of Internal Medicine, Sungkyunkwan University School of Medicine, Kangbuk Samsung Hospital, Seoul, Korea. diksmc.park@samsung.com
- 3Department of Internal Medicine, Inje University College of Medicine, Haeundae Paik Hospital, Busan, Korea.
- 4Department of Internal Medicine, Inje University College of Medicine, Seoul Paik Hospital, Seoul, Korea.
- 5Department of Internal Medicine, Soonchunhyang University College of Medicine, Soonchunhyang University Cheonan Hospital, Cheonan, Korea.
- 6Department of Internal Medicine, Seoul National University Boramae Hospital, Seoul, Korea.
- 7Department of Internal Medicine, Dongguk University Ilsan Hospital, Goyang, Korea.
- 8Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea.
- KMID: 2174380
- DOI: http://doi.org/10.5217/ir.2014.12.3.214
Abstract
- BACKGROUND/AIMS
Infliximab was introduced recently as a rescue therapy for ulcerative colitis (UC) patients refractory to conventional treatments such as therapy with 5-amiono salicylic acids (5-ASA), immune modulators, and corticosteroids. However, there is insufficient data about its efficacy and safety in Korea.
METHODS
From 7 tertiary referral hospitals, 33 patients who were treated with infliximab for moderate to severe (Mayo score 6-12) UC refractory to conventional treatment were recruited to this study. Clinical remission was defined as a total Mayo score of 2 or lower and every subscore less than 2. Partial response was defined as a decrease of Mayo score at least 3 points from baseline.
RESULTS
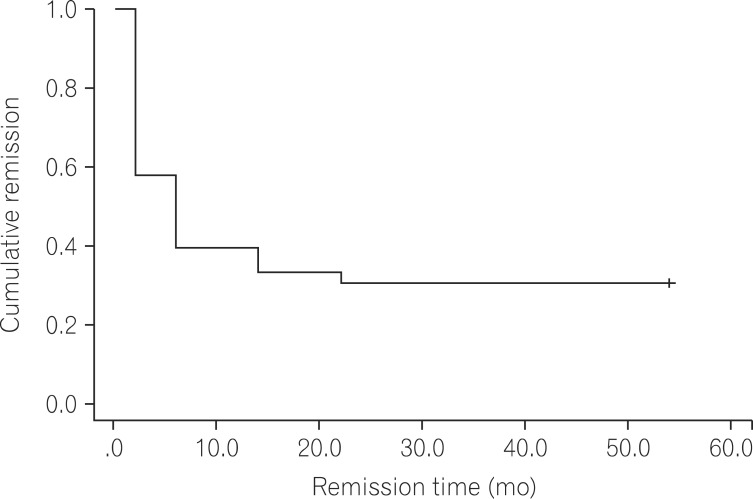
Twenty-three patients (69.7%) showed clinical remission and 29 patients (87.8%) showed partial response in the observation period. When the remission and non-remission groups were compared in univariate analysis, only a higher total Mayo score at base line (11.0+/-0.9 vs. 9.9+/-1.5; P=0.04) was related to remission. The remission maintenance rate decreased with time in the Kaplan-Meier analysis. Two patients experienced re-remission after the first remission followed by aggravation during infliximab treatment. Three patients stopped infliximab treatment owing to adverse events including rhabdomyolysis, pneumonia, and fever of unknown origin.
CONCLUSIONS
If there is no choice except surgery for UC patients refractory to conventional treatment, infliximab is an effective and relatively safe treatment option for these patients in Korea.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
How to Write Bibliographies for Citing Domestic Academic Resources
Hyun Jung Yi
Korean J Gastroenterol. 2015;65(1):70-72. doi: 10.4166/kjg.2015.65.1.70.Circulating Ghrelin Levels and Obestatin/Ghrelin Ratio as a Marker of Activity in Ulcerative Colitis
Ja Young Jung, Ji Bong Jeong, Ji Won Kim, Su Hwan Kim, Seong-Joon Koh, Byeong Gwan Kim, Kook Lae Lee
Intest Res. 2015;13(1):68-73. doi: 10.5217/ir.2015.13.1.68.Inflammatory Bowel Disease Cohort Studies in Korea: Present and Future
Jung Won Lee, Jong Pil Im, Jae Hee Cheon, You Sun Kim, Joo Sung Kim, Dong Soo Han
Intest Res. 2015;13(3):213-218. doi: 10.5217/ir.2015.13.3.213.
Reference
-
1. Staehelin A. Prognosis of ulcerative colitis. Follow-up control of cases from 1942 to 1969. Schweiz Med Wochenschr. 1970; 100:580–582. PMID: 5420854.2. Kim SJ, Cho YK, Rhee JE, Yoon CM. Two cases of ulcerative colitis. Korean J Gastroenterol. 1970; 2:29–33.3. Yang SK, Hong WS, Min YI, et al. Incidence and prevalence of ulcerative colitis in the Songpa-Kangdong District, Seoul, Korea, 1986-1997. J Gastroenterol Hepatol. 2000; 15:1037–1042. PMID: 11059934.
Article4. Choi CH, Kim YH, Kim YS, et al. Guidelines for the management of ulcerative colitis. Korean J Gastroenterol. 2012; 59:118–140. PMID: 22387836.
Article5. Wada Y, Matsui T, Matake H, et al. Intractable ulcerative colitis caused by cytomegalovirus infection: a prospective study on prevalence, diagnosis, and treatment. Dis Colon Rectum. 2003; 46:S59–S65. PMID: 14530660.6. Criscuoli V, Casa A, Orlando A, et al. Severe acute colitis associated with CMV: a prevalence study. Dig Liver Dis. 2004; 36:818–820. PMID: 15646428.
Article7. Kim YS, Kim YH, Kim JS, et al. The prevalence and efficacy of ganciclovir on steroid-refractory ulcerative colitis with cytomegalovirus infection: a prospective multicenter study. J Clin Gastroenterol. 2012; 46:51–56. PMID: 21552140.
Article8. Van Assche G, D'Haens G, Noman M, et al. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology. 2003; 125:1025–1031. PMID: 14517785.
Article9. Campbell S, Travis S, Jewell D. Ciclosporin use in acute ulcerative colitis: a long-term experience. Eur J Gastroenterol Hepatol. 2005; 17:79–84. PMID: 15647646.10. Haslam N, Hearing SD, Probert CS. Audit of cyclosporin use in inflammatory bowel disease: limited benefits, numerous side-effects. Eur J Gastroenterol Hepatol. 2000; 12:657–660. PMID: 10912486.
Article11. Knight DM, Trinh H, Le J, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol. 1993; 30:1443–1453. PMID: 8232330.
Article12. Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993; 34:1705–1709. PMID: 8031350.
Article13. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005; 353:2462–2476. PMID: 16339095.
Article14. Lee KM, Jeen YT, Cho JY, et al. Efficacy, safety, and predictors of response to infliximab therapy for ulcerative colitis: a Korean multicenter retrospective study. J Gastroenterol Hepatol. 2013; 28:1829–1833. PMID: 23829336.
Article15. Jurgens M, Laubender RP, Hartl F, et al. Disease activity, ANCA, and IL23R genotype status determine early response to infliximab in patients with ulcerative colitis. Am J Gastroenterol. 2010; 105:1811–1819. PMID: 20197757.
Article16. Oussalah A, Evesque L, Laharie D, et al. A multicenter experience with infliximab for ulcerative colitis: outcomes and predictors of response, optimization, colectomy, and hospitalization. Am J Gastroenterol. 2010; 105:2617–2625. PMID: 20736936.
Article17. Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010; 59:49–54. PMID: 19651627.
Article18. Park SH, Yang SK, Hong SM, et al. Severe disease activity and cytomegalovirus colitis are predictive of a nonresponse to infliximab in patients with ulcerative colitis. Dig Dis Sci. 2013; 58:3592–3599. PMID: 23979435.
Article19. Chaparro M, Burgueno P, Iglesias E, et al. Infliximab salvage therapy after failure of ciclosporin in corticosteroid-refractory ulcerative colitis: a multicentre study. Aliment Pharmacol Ther. 2012; 35:275–283. PMID: 22142227.
Article20. Sjoberg M, Walch A, Meshkat M, et al. Infliximab or cyclosporine as rescue therapy in hospitalized patients with steroid-refractory ulcerative colitis: a retrospective observational study. Inflamm Bowel Dis. 2012; 18:212–218. PMID: 21438096.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Type II Segmental Vitiligo Developed under Infliximab Treatment for Ulcerative Colitis
- Optic Neuritis after Infliximab Treatment in a Patient with Ulcerative Colitis
- Golimumab Therapy in Ulcerative Colitis
- What to do when traditional rescue therapies fail in acute severe ulcerative colitis
- Advances in ulcerative colitis therapy