Diabetes Metab J.
2013 Jun;37(3):176-180. 10.4093/dmj.2013.37.3.176.
An In Vitro Model to Probe the Regulation of Adipocyte Differentiation under Hyperglycemia
- Affiliations
-
- 1Centre for Biotechnology, Anna University, Chennai, India. lakshmibs@annauniv.edu
- KMID: 2174291
- DOI: http://doi.org/10.4093/dmj.2013.37.3.176
Abstract
- BACKGROUND
The aim of this study was an in vitro investigation of the effect of high glucose concentration on adipogenesis, as prolonged hyperglycemia alters adipocyte differentiation.
METHODS
3T3-L1 preadipocytes differentiated in the presence of varying concentrations of glucose (25, 45, 65, 85, and 105 mM) were assessed for adipogenesis using AdipoRed (Lonza) assay. Cell viability and proliferation were measured using MTT reduction and [3H] thymidine incorporation assay. The extent of glucose uptake and glycogen synthesis were measured using radiolabelled 2-deoxy-D-[1-3H] glucose and [14C]-UDP-glucose. The gene level expression was evaluated using reverse transcription-polymerase chain reaction and protein expression was studied using Western blot analysis.
RESULTS
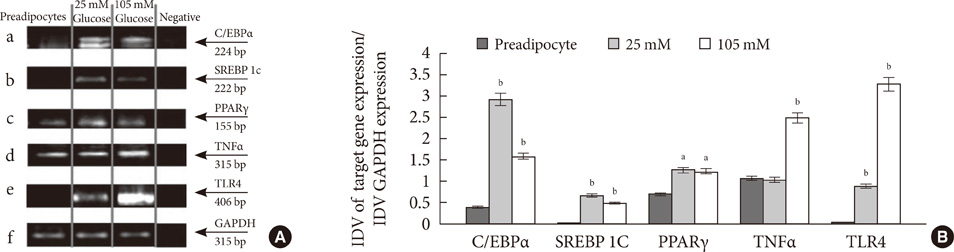
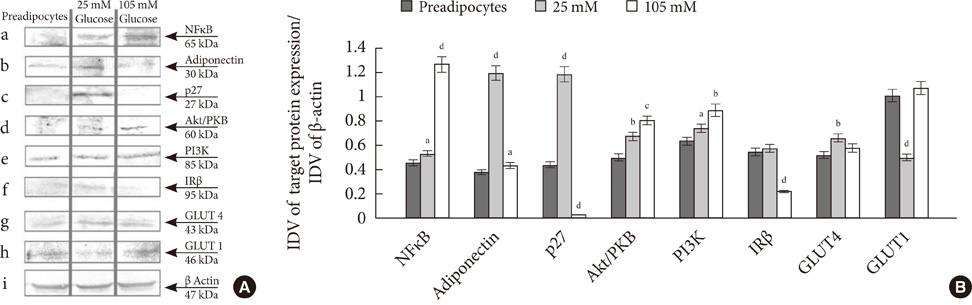
Glucose at 105 mM concentration was observed to inhibit adipogenesis through inhibition of CCAAT-enhancer-binding proteins, sterol regulatory element-binding protein, peroxisome proliferator-activated receptor and adiponectin. High concentration of glucose induced stress by increasing levels of toll-like receptor 4, nuclear factor kappaB and tumor necrosis factor alpha thereby generating activated preadipocytes. These cells entered the state of hyperplasia through inhibition of p27 and proliferation was found to increase through activation of protein kinase B via phosphoinositide 3 kinase dependent pathway. This condition inhibited insulin signaling through decrease in insulin receptor beta. Although the glucose transporter 4 (GLUT4) protein remained unaltered with the glycogen synthesis inhibited, the cells were found to exhibit an increase in glucose uptake via GLUT1.
CONCLUSION
Adipogenesis in the presence of 105 mM glucose leads to an uncontrolled proliferation of activated preadipocytes providing an insight towards understanding obesity.
Keyword
MeSH Terms
-
Adipocytes
Adipogenesis
Adiponectin
Blotting, Western
CCAAT-Enhancer-Binding Proteins
Cell Survival
Glucose
Glucose Transport Proteins, Facilitative
Glycogen
Hyperglycemia
Hyperplasia
Insulin
Obesity
Peroxisomes
Phosphotransferases
Proto-Oncogene Proteins c-akt
Receptor, Insulin
Thymidine
Toll-Like Receptor 4
Tumor Necrosis Factor-alpha
Adiponectin
CCAAT-Enhancer-Binding Proteins
Glucose
Glucose Transport Proteins, Facilitative
Glycogen
Insulin
Phosphotransferases
Proto-Oncogene Proteins c-akt
Receptor, Insulin
Thymidine
Toll-Like Receptor 4
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Yue T, Yin J, Li F, Li D, Du M. High glucose induces differentiation and adipogenesis in porcine muscle satellite cells via mTOR. BMB Rep. 2010; 43:140–145.2. Aguiari P, Leo S, Zavan B, Vindigni V, Rimessi A, Bianchi K, Franzin C, Cortivo R, Rossato M, Vettor R, Abatangelo G, Pozzan T, Pinton P, Rizzuto R. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci U S A. 2008; 105:1226–1231.3. Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007; 56:1517–1526.4. Gustafson B, Gogg S, Hedjazifar S, Jenndahl L, Hammarstedt A, Smith U. Inflammation and impaired adipogenesis in hypertrophic obesity in man. Am J Physiol Endocrinol Metab. 2009; 297:E999–E1003.5. Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 2009; 58:1550–1557.6. Charriere G, Cousin B, Arnaud E, Andre M, Bacou F, Penicaud L, Casteilla L. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003; 278:9850–9855.7. Shilpa K, Sangeetha KN, Muthusamy VS, Sujatha S, Lakshmi BS. Probing key targets in insulin signaling and adipogenesis using a methanolic extract of Costus pictus and its bioactive molecule, methyl tetracosanoate. Biotechnol Lett. 2009; 31:1837–1841.8. Sangeetha KN, Sujatha S, Muthusamy VS, Anand S, Nithya N, Velmurugan D, Balakrishnan A, Lakshmi BS. 3beta-taraxerol of Mangifera indica, a PI3K dependent dual activator of glucose transport and glycogen synthesis in 3T3-L1 adipocytes. Biochim Biophys Acta. 2010; 1800:359–366.9. Sathya S, Sudhagar S, Vidhya Priya M, Bharathi Raja R, Muthusamy VS, Niranjali Devaraj S, Lakshmi BS. 3beta-hydroxylup-20(29)-ene-27,28-dioic acid dimethyl ester, a novel natural product from Plumbago zeylanica inhibits the proliferation and migration of MDA-MB-231 cells. Chem Biol Interact. 2010; 188:412–420.10. Ramadevi Mani S, Lakshmi BS. G1 arrest and caspase-mediated apoptosis in HL-60 cells by dichloromethane extract of Centrosema pubescens. Am J Chin Med. 2010; 38:1143–1159.11. Han CY, Subramanian S, Chan CK, Omer M, Chiba T, Wight TN, Chait A. Adipocyte-derived serum amyloid A3 and hyaluronan play a role in monocyte recruitment and adhesion. Diabetes. 2007; 56:2260–2273.12. Ferrante AW Jr. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007; 262:408–414.13. Nadler ST, Attie AD. Please pass the chips: genomic insights into obesity and diabetes. J Nutr. 2001; 131:2078–2081.14. Naaz A, Holsberger DR, Iwamoto GA, Nelson A, Kiyokawa H, Cooke PS. Loss of cyclin-dependent kinase inhibitors produces adipocyte hyperplasia and obesity. FASEB J. 2004; 18:1925–1927.15. Auld CA, Morrison RF. Evidence for cytosolic p27(Kip1) ubiquitylation and degradation during adipocyte hyperplasia. Obesity (Silver Spring). 2006; 14:2136–2144.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Response: An In Vitro Model to Probe the Regulation of Adipocyte Differentiation under Hyperglycemia (Diabetes Metab J 2013;37:176-80)
- Letter: An In Vitro Model to Probe the Regulation of Adipocyte Differentiation under Hyperglycemia (Diabetes Metab J 2013;37:176-80)
- Transcriptional repression of type I procollagen genes during adipocyte differentiation
- Regulation of Adipocyte Differentiation via MicroRNAs
- Effects of (6)-gingerol, ginger component on adipocyte development and differentiation in 3T3-L1