J Gynecol Oncol.
2011 Dec;22(4):233-238. 10.3802/jgo.2011.22.4.233.
Synchronous gynecologic malignancy and preliminary results of Lynch syndrome
- Affiliations
-
- 1Division of Gynecologic Oncology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- 2Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Experimental Pathology Center, Samsung Cancer Research Institute, Seoul, Korea.
- 4Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. bgkim@skku.edu
- KMID: 2173624
- DOI: http://doi.org/10.3802/jgo.2011.22.4.233
Abstract
OBJECTIVE
Lynch syndrome is a hereditary cancer syndrome that increases the risks of colorectal and gynecologic malignancies such as endometrial and ovarian cancer. Studies have shown that mutations in mismatch repair genes (MSH2, MSH6, and MLH1) are associated with Lynch syndrome. The aim of our study was to estimate the value of MSH2, MSH6, and MLH1 immunohistochemistry based on family history in a Korean sample.
METHODS
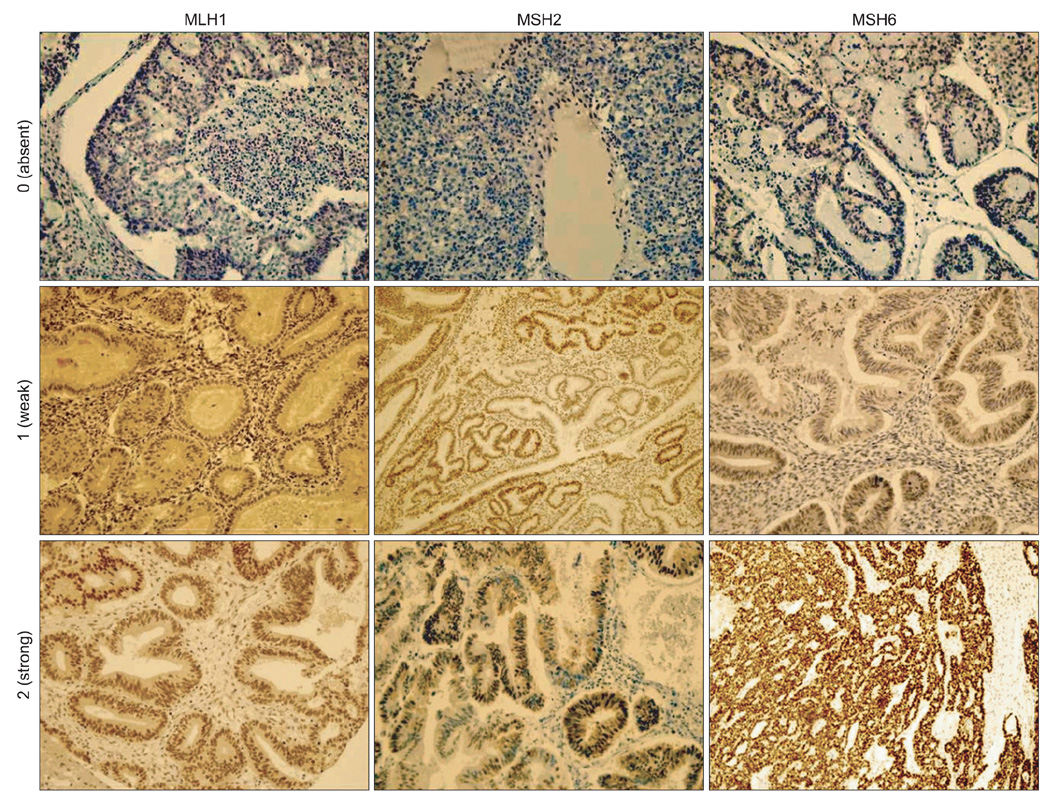
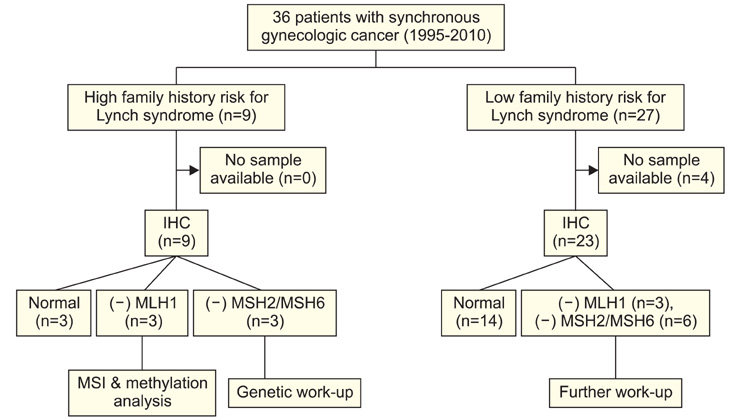
Thirty six women with synchronous gynecologic tumors of endometrial and ovarian cancer were identified among patients being treated at our institution. Among them, 32 patients had tumor blocks (total 62 slides) available for analysis. According to a diagnostic algorithm, we performed immunohistochemistry analyses. Staining was scored based on intensity and proportion (negative or 0: intensity undetectable or minimal, proportion <5%; weak or 1+: intensity mild, proportion 5-30%; strong or 2+: intensity moderate to marked, proportion 30-99%).
RESULTS
Among 32 eligible patients, 9 (28%) had a family history of cancer. Six patients (19%) were negative for MLH1; among them, four (4/6) were negative at both sites. Nine patients (28%) were negative for MSH2 or MSH6 at both sites or negative for both MSH2 and MSH6. Among these three patients showed negative staining for both sites. The three patients showing negative staining for MLH1, MSH2, and MSH6 at both sites with family history were considered to be the screening positive groups of Lynch syndrome.
CONCLUSION
In this study, the frequency of Lynch syndrome associated immunohistochemical staining (MLH1, MSH2, and MSH6) group was estimated as 9% (3/32) among Korean women with synchronous gynecologic tumors.
MeSH Terms
Figure
Cited by 1 articles
-
Screening for Lynch syndrome using risk assessment criteria in patients with ovarian cancer
Takashi Takeda, Kosuke Tsuji, Kouji Banno, Megumi Yanokura, Yusuke Kobayashi, Eiichiro Tominaga, Daisuke Aoki
J Gynecol Oncol. 2018;29(3):. doi: 10.3802/jgo.2018.29.e29.
Reference
-
1. Lynch HT, Shaw MW, Magnuson CW, Larsen AL, Krush AJ. Hereditary factors in cancer: study of two large midwestern kindreds. Arch Intern Med. 1966. 117:206–212.2. Fishel R, Lescoe MK, Rao MR, Copeland NG, Jenkins NA, Garber J, et al. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993. 75:1027–1038.3. Papadopoulos N, Nicolaides NC, Wei YF, Ruben SM, Carter KC, Rosen CA, et al. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994. 263:1625–1629.4. Leach FS, Nicolaides NC, Papadopoulos N, Liu B, Jen J, Parsons R, et al. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993. 75:1215–1225.5. Bronner CE, Baker SM, Morrison PT, Warren G, Smith LG, Lescoe MK, et al. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature. 1994. 368:258–261.6. Burgart LJ. Testing for defective DNA mismatch repair in colorectal carcinoma: a practical guide. Arch Pathol Lab Med. 2005. 129:1385–1389.7. Hendriks YM, Jagmohan-Changur S, van der Klift HM, Morreau H, van Puijenbroek M, Tops C, et al. Heterozygous mutations in PMS2 cause hereditary nonpolyposis colorectal carcinoma (Lynch syndrome). Gastroenterology. 2006. 130:312–322.8. Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999. 116:1453–1456.9. Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004. 96:261–268.10. Dunlop MG, Farrington SM, Carothers AD, Wyllie AH, Sharp L, Burn J, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997. 6:105–110.11. Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, de la Chapelle A, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999. 81:214–218.12. Soliman PT, Broaddus RR, Schmeler KM, Daniels MS, Gonzalez D, Slomovitz BM, et al. Women with synchronous primary cancers of the endometrium and ovary: do they have Lynch syndrome? J Clin Oncol. 2005. 23:9344–9350.13. Walsh CS, Blum A, Walts A, Alsabeh R, Tran H, Koeffler HP, et al. Lynch syndrome among gynecologic oncology patients meeting Bethesda guidelines for screening. Gynecol Oncol. 2010. 116:516–521.14. Yoon SN, Ku JL, Shin YK, Kim KH, Choi JS, Jang EJ, et al. Hereditary nonpolyposis colorectal cancer in endometrial cancer patients. Int J Cancer. 2008. 122:1077–1081.15. Lim MC, Seo SS, Kang S, Seong MW, Lee BY, Park SY. Hereditary non-polyposis colorectal cancer/Lynch syndrome in Korean patients with endometrial cancer. Jpn J Clin Oncol. 2010. 40:1121–1127.16. Johnson CH. SEER program coding and staging manual 2004. Rev. 1. 2004. Bethesda, MD: National Cancer Institute.17. Scully RE, Young RH, Clement PB. Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament. Atlas of tumor pathology. 1998. Bethesda, MD: Armed Forces Institute of Pathology.18. Koornstra JJ, Mourits MJ, Sijmons RH, Leliveld AM, Hollema H, Kleibeuker JH. Management of extracolonic tumours in patients with Lynch syndrome. Lancet Oncol. 2009. 10:400–408.19. Evans-Metcalf ER, Brooks SE, Reale FR, Baker SP. Profile of women 45 years of age and younger with endometrial cancer. Obstet Gynecol. 1998. 91:349–354.20. Soliman PT, Oh JC, Schmeler KM, Sun CC, Slomovitz BM, Gershenson DM, et al. Risk factors for young premenopausal women with endometrial cancer. Obstet Gynecol. 2005. 105:575–580.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lynch syndrome – Muir-Torre variant: implication in gynecologic oncology
- Early onset of colorectal cancer in a 13-year-old girl with Lynch syndrome
- Screening for Lynch syndrome using risk assessment criteria in patients with ovarian cancer
- Colonic Adenoma Characteristics in Gynecologic Cancer Patients
- Hereditary Colon Cancer: Lynch Syndrome