J Gynecol Oncol.
2010 Mar;21(1):38-44. 10.3802/jgo.2010.21.1.38.
Proliferation of CD4(+)CD25(high+)Foxp3(+) regulatory T lymphocytes in ex vivo expanded ascitic fluid from primary and recurrent ovarian carcinoma
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. ymkim@amc.seoul.kr
- 2Department of Medicine, University of Ulsan The Graduate School, Seoul, Korea.
- KMID: 2173510
- DOI: http://doi.org/10.3802/jgo.2010.21.1.38
Abstract
OBJECTIVE
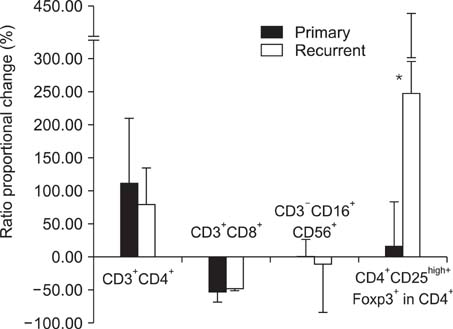
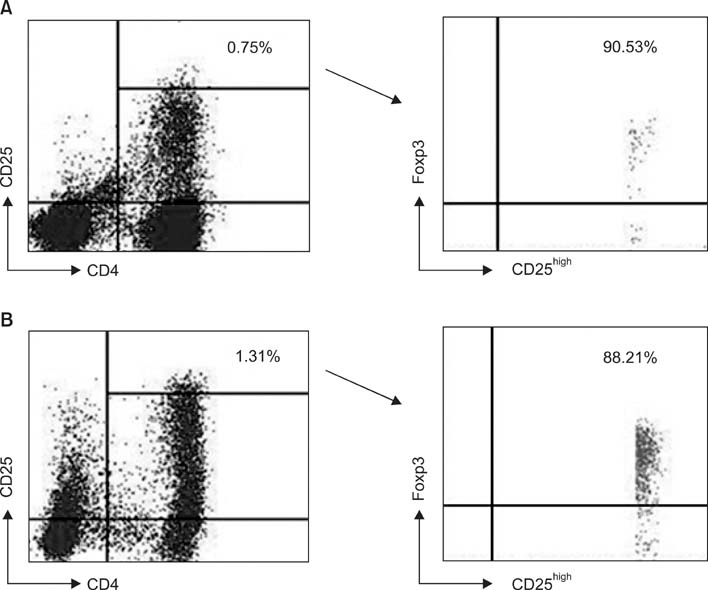
Regulatory T lymphocytes evoke the immune tolerance by suppressing and inactivating cytotoxic T lymphocytes. The objective of this study was to compare the proportion of regulatory T lymphocytes, precisely defined as CD4(+)CD25(high+)Foxp3(+) T lymphocytes, in primary and recurrent ovarian carcinoma before and after ex vivo expansion of ascites with interleukin-2 (IL-2). METHODS: Ascitic fluid samples were obtained from 26 patients with ovarian carcinoma. Lymphocytes were isolated from ascites and cell markers were analyzed by flow cytometry using anti-CD3/CD4/CD8/CD16/CD56/CD25 and anti-Foxp3 antibodies. Lymphocytes were incubated for 2 to 3 weeks and expanded ex vivo by IL-2 stimulation and their phenotypes were analyzed by flow cytometry. RESULTS: Following ex vivo expansion, ascitic fluid lymphocytes increased by a greater extent in the recurrent group than in the primary group. The proportion of ex vivo-expanded lymphocytes changed as follows; CD4(+) T lymphocytes increased, CD8(+) T lymphocytes decreased, and the proportion of CD3(-)CD16(+)56(+) NK cells was unchanged. The proportion of CD4(+)CD25(high+)Foxp3(+) regulatory T lymphocytes in CD4(+) T lymphocytes increased after ex vivo expansion in both groups, but to a greater degree in the recurrent group. CONCLUSION: This study showed that regulatory T lymphocytes, neither cytotoxic T lymphocytes nor NK cells, were extensively increased after ex vivo expansion, especially in recurrent ovarian carcinoma. These results may provide information that helps to guide the future development of adoptive immunotherapy against ovarian carcinoma.
MeSH Terms
Figure
Reference
-
1. Bremers AJ, Parmiani G. Immunology and immunotherapy of human cancer: present concepts and clinical developments. Crit Rev Oncol Hematol. 2000. 34:1–25.2. Berek JS, Schultes BC, Nicodemus CF. Biologic and immunologic therapies for ovarian cancer. J Clin Oncol. 2003. 21:10 Suppl. 168s–174s.3. O'Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004. 10:801–805.4. Thompson C, Powrie F. Regulatory T cells. Curr Opin Pharmacol. 2004. 4:408–414.5. Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004. 4:841–855.6. Mittrucker HW, Kaufmann SH. Mini-review: regulatory T cells and infection: suppression revisited. Eur J Immunol. 2004. 34:306–312.7. Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, et al. Immunologic tolerance maintained by CD25+CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001. 182:18–32.8. Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003. 9:606–612.9. Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J Exp Med. 2000. 192:295–302.10. Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002. 168:1080–1086.11. Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003. 4:337–342.12. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003. 299:1057–1061.13. Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004. 10:942–949.14. Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002. 169:2756–2761.15. Freedman RS, Tomasovic B, Templin S, Atkinson EN, Kudelka A, Edwards CL, et al. Large-scale expansion in interleukin-2 of tumor-infiltrating lymphocytes from patients with ovarian carcinoma for adoptive immunotherapy. J Immunol Methods. 1994. 167:145–160.16. Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003. 98:1089–1099.17. Elgert KD, Alleva DG, Mullins DW. Tumor-induced immune dysfunction: the macrophage connection. J Leukoc Biol. 1998. 64:275–290.18. Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001. 193:1285–1294.19. Ochsenbein AF, Sierro S, Odermatt B, Pericin M, Karrer U, Hermans J, et al. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001. 411:1058–1064.20. Kono K, Salazar-Onfray F, Petersson M, Hansson J, Masucci G, Wasserman K, et al. Hydrogen peroxide secreted by tumor-derived macrophages down-modulates signal-transducing zeta molecules and inhibits tumor-specific T cell-and natural killer cell-mediated cytotoxicity. Eur J Immunol. 1996. 26:1308–1313.21. Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970. 18:723–737.22. Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002. 2:389–400.23. Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25 high regulatory cells in human peripheral blood. J Immunol. 2001. 167:1245–1253.24. Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005. 6:331–337.25. Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999. 59:3128–3133.26. Rosenberg SA. Immunotherapy and gene therapy of cancer. Cancer Res. 1991. 51:18 Suppl. 5074s–5079s.27. Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, et al. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002. 168:4272–4276.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Proliferation of CD4(+)CD25(high+)Foxp3(+) regulatory T lymphocytes in ex vivo expanded ascitic fluid from primary and recurrent ovarian carcinoma
- Peripheral Generation of CD4+ CD25+ Foxp3+ Regulatory T Cells
- A Study on the Number of Circulating CD4+CD25+Foxp3+ Regulatory T Cells and CD4+CD25-Foxp3+ T Cells in Psoriasis
- Dynamic Frequency of Blood CD4+CD25+ Regulatory T Cells in Rats with Collagen-induced Arthritis
- Characterization of regulatory T cells in ex vivo expansion of human umbilical cord blood