J Gynecol Oncol.
2008 Sep;19(3):157-161. 10.3802/jgo.2008.19.3.157.
Present status and future direction of clinical trials in advanced endometrial carcinoma
- Affiliations
-
- 1Division of Gynecologic Oncology, Brody School of Medicine at East Carolina University, Greenville, NC, USA. homesleyh@ecu.edu
- KMID: 2173395
- DOI: http://doi.org/10.3802/jgo.2008.19.3.157
Abstract
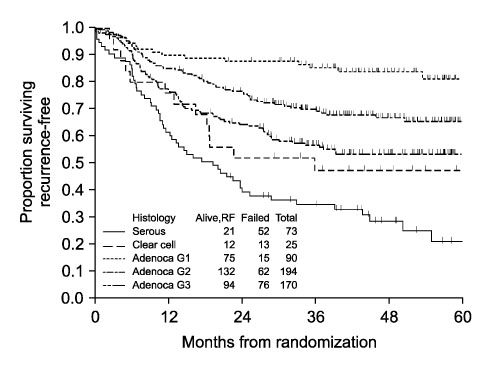
- Endometrial adenocarcinoma is staged surgically, and advanced endometrial carcinoma is considered to be FIGO stage III and IV. The Gynecologic Oncology Group (GOG) has come a long way in developing new strategies in the management of advanced endometrial carcinoma. Combining surgery, radiation, and chemotherapy, the 5-year survival has improved to between 40-60% in newly diagnosed advanced endometrial carcinoma. Recent findings in GOG184 indicate that multiple risk factors noted at the time of surgical staging could lead to concurrent clinical trials that could be completed expeditiously rather than a subsequent ten year long phase III trial including all the various risk subgroups of patients. This review is a focus on the accomplishments of the GOG in advanced endometrial carcinoma with an emphasis on future challenges.
Keyword
Figure
Reference
-
1. Homesley HD, Filiaci V, Gibbons SK, Long HJ, Spirtos NM, Morris RT, et al. Randomized phase III trial in advanced endometrial carcinoma of surgery and volume-directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: a Gynecologic Oncology Group study. Gynecol Oncol. 2008. 108:S2.2. Gibbons S, Martinez A, Schray M, Podratz K, Stanhope R, Garton G, et al. Adjuvant whole abdominopelvic irradiation for high risk endometrial carcinoma. Int J Radiat Oncol Biol Phys. 1991. 21:1019–1025.3. Reisinger SA, Asbury R, Liao SY, Homesley HD. A phase I study of weekly cisplatin and whole abdominal radiation for the treatment of stage III and IV endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 1996. 63:299–303.4. Soper JT, Reisinger SA, Ashbury R, Jones E, Clarke-Pearson DL. Feasibility study of concurrent weekly cisplatin and whole abdominopelvic irradiation followed by doxorubicin/cisplatin chemotherapy for advanced stage endometrial carcinoma: a Gynecologic Oncology Group trial. Gynecol Oncol. 2004. 95:95–100.5. Sutton G, Axelrod JH, Bundy BN, Roy T, Homesley H, Lee RB, et al. Adjuvant whole abdominal irradiation in clinical stages I and II papillary serous or clear cell carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 2006. 100:349–354.6. Thigpen JT, Brady MF, Homesley HD, Malfetano J, DuBeshter B, Burger RA, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2004. 22:3902–3908.7. Lincoln S, Blessing JA, Lee RB, Rocereto TF. Activity of paclitaxel as second-line chemotherapy in endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2003. 88:277–281.8. Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006. 24:36–44.9. Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004. 22:2159–2166.