Current status of gynecologic cancer in Japan
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Kurume University School of Medicine, Kurume, Japan. kimi@med.kurume-u.ac.jp
- KMID: 2245082
- DOI: http://doi.org/10.3802/jgo.2009.20.2.67
Abstract
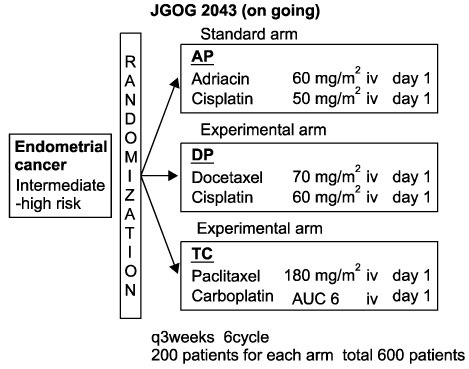
- To make an overview of the current status of gynecologic cancer in Japan, we reviewed the recent incidence of cervical, endometrial, and ovarian cancer in Japanese women. The incidence of all three cancers has increased, but trends differ respectively. In age specific cancer site distribution data, the uterus and ovary are leading sites of high incidence among Japanese women younger than 40 years of age. Therefore, fertility sparing cancer treatment has received much attention. Several multicenter clinical trials have been done by Japanese groups, and some excellent evidence has been collected for endometrial and ovarian cancer. A promising international collaboration trial for ovarian clear cell carcinoma is also underway at the present time.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Assessing the effect of guideline introduction on clinical practice and outcome in patients with endometrial cancer in Japan: a project of the Japan Society of Gynecologic Oncology (JSGO) guideline evaluation committee
Shogo Shigeta, Satoru Nagase, Mikio Mikami, Masae Ikeda, Masako Shida, Isao Sakaguchi, Norichika Ushioda, Fumiaki Takahashi, Wataru Yamagami, Nobuo Yaegashi, Yasuhiro Udagawa, Hidetaka Katabuchi
J Gynecol Oncol. 2017;28(6):e76. doi: 10.3802/jgo.2017.28.e76.Assessing the effect of guideline introduction on clinical practice and outcome in patients with endometrial cancer in Japan: a project of the Japan Society of Gynecologic Oncology (JSGO) guideline evaluation committee
Shogo Shigeta, Satoru Nagase, Mikio Mikami, Masae Ikeda, Masako Shida, Isao Sakaguchi, Norichika Ushioda, Fumiaki Takahashi, Wataru Yamagami, Nobuo Yaegashi, Yasuhiro Udagawa, Hidetaka Katabuchi
J Gynecol Oncol. 2017;28(6):. doi: 10.3802/jgo.2017.28.e76.
Reference
-
1. Oshima A, Kuroisi T, Tajima K. Cancer statistic statement. 2004. Tokyo: Shinohara Publishing.2. Report from gynecologic tumor committee of JSOG. Acta Obstet Gynaecol Jpn. 2007. 59:952.3. Report from gynecologic tumor committee of JSOG. Acta Obstet Gynaecol Jpn. 2009. 61:936.4. Report from gynecologic tumor committee of JSOG. Acta Obstet Gynaecol Jpn. 2000. 52:855.5. Kaku T, Yoshikawa H, Tsuda H, Sakamoto A, Fukunaga M, Kamura T, et al. Conservative therapy for adenocarcinoma and atypical endometrial hyperplasia of the endometrium in young women: central pathologic review and treatment outcome. Cancer Lett. 2001. 167:39–48.6. Ushijima K, Yahata H, Yoshikawa H, Konishi I, Yasugi T, Saito T, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007. 25:2798–2803.7. Japan Society of Gynecologic Oncology. Ovarian cancer treatment guidelines 2007. 2007. Tokyo: Kanehara & Co. Ltd.8. Susumu N, Sagae S, Udagawa Y, Niwa K, Kuramoto H, Satoh S, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008. 108:226–233.9. Rose PG, Smrekar M, Fusco N. A phase II trial of weekly paclitaxel and every 3 weeks of carboplatin in potentially platinum-sensitive ovarian and peritoneal carcinoma. Gynecol Oncol. 2005. 96:296–300.10. Isonishi S, Yasuda M, Takahashi F, Katsumata N, Kimura E, Aoki D, et al. Randomized phase III trial of conventional paclitaxel and carboplatin (c-TC) versus dose dense weekly paclitaxel and carboplatin (dd-TC) in women with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer: Japanese Gynecologic Oncology [abstract]. J Clin Oncol. 2008. 26(15S):abstr 5506.11. Taguchi K. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000. 88:2584–2589.12. Enomoto T, Kuragaki C, Yamasaki M, Sugita N, Otsuki Y, Ikegami H, et al. Is clear cell carcinoma and mucinous carcinoma of the ovary sensitive to combination chemotherapy with paclitaxel and carboplatin? [abstract]. Proc Am Soc Clin Oncol. 2003. 22:abstr 1797.13. Sugiyama T, Yakushiji M, Nishida T, Ushijima K, Okura N, Kigawa J, et al. Irinotecan (CPT-11) combined with cisplatin in patients with refractory or recurrent ovarian cancer. Cancer Lett. 1998. 128:211–218.14. Takano M, Kikuchi Y, Yaegashi N, Suzuki M, Tsuda H, Sagae S, et al. Adjuvant chemotherapy with irinotecan hydrochloride and cisplatin for clear cell carcinoma of the ovary. Oncol Rep. 2006. 16:1301–1306.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current status and future directions of ovarian cancer prognostic models
- Annual report of gynecologic cancer registry program in Korea: 1991~2004
- Towards the elimination of cervical cancer in Japan
- Current status of hereditary breast and ovarian cancer practice among gynecologic oncologists in Japan: a nationwide survey by the Japan Society of Gynecologic Oncology (JSGO)
- The current status of laparoscopic and robotic para-aortic lymphadenectomy in gynecologic cancer surgery