Blood Res.
2013 Jun;48(2):121-127. 10.5045/br.2013.48.2.121.
CD163 and c-Met expression in the lymph node and the correlations between elevated levels of serum free light chain and the different clinicopathological parameters of advanced classical Hodgkin's lymphoma
- Affiliations
-
- 1Military Medical Academy, Cairo, Egypt. dr_ahmed_bedewy@yahoo.com
- 2Hematology Department, Medical Research Institute, Alexandria, Egypt.
- KMID: 2172910
- DOI: http://doi.org/10.5045/br.2013.48.2.121
Abstract
- BACKGROUND
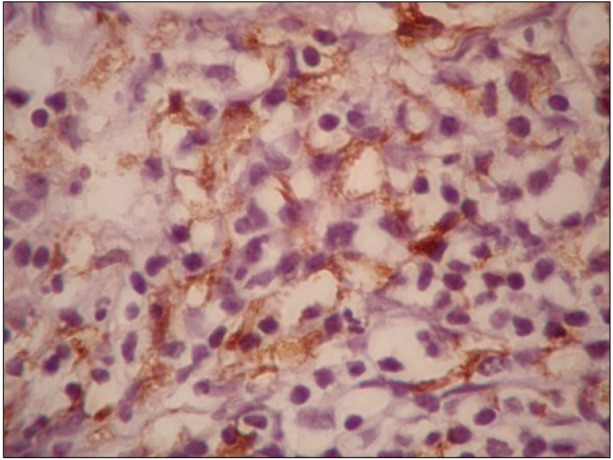
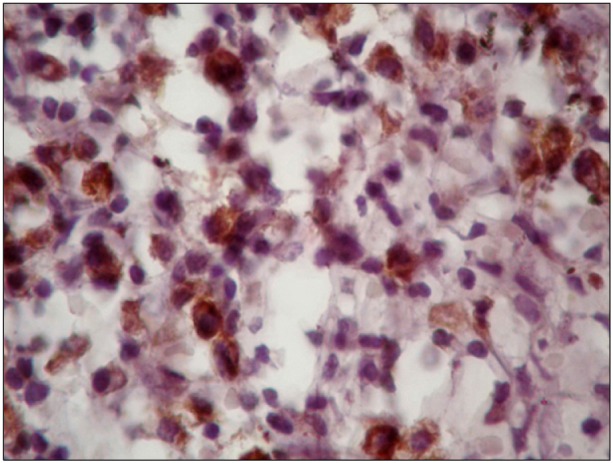
Advances in the understanding of Hodgkin's lymphoma (HL) show various functions of infiltrating immune cells and cytokines in relation to clinical outcomes. The expression of CD163 and c-Met has been suggested to have a role in lymphoid malignancy. Thus, we evaluated the expressions of CD163, c-Met, and serum free light chain (sFLC) in relation to the clinicopathological features of patients with advanced classical HL (cHL).
METHODS
We assessed the expression of CD163 and c-Met in 34 patients with cHL through immunohistochemistry on the lymph node biopsy sections and the levels of pretreatment sFLC were estimated using ELISA.
RESULTS
High CD163 expression correlated with increased age, B symptoms, International Prognostic Score (IPS) > or =3, mixed cellularity subtype, and low response to treatment. Further, high c-Met expression correlated with increased age at diagnosis, leukocytosis, B symptoms, and lower chance to achieve complete remission. The sFLC levels correlated with increased age at diagnosis, lymphopenia, IPS > or =3, B symptoms, and lower complete remission rates.
CONCLUSION
In advanced cHL, increased expression of CD163 and c-Met showed a significant association with adverse prognostic parameters and poor response to treatment. Pretreatment high sFLC level also correlated with poor risk factors, suggesting its use as a candidate prognostic marker. A comprehensive approach for prognostic markers might represent a step towards developing a tailored therapeutic approach for HL.
Keyword
MeSH Terms
Figure
Reference
-
1. Aleman BM, van den Belt-Dusebout AW, Klokman WJ, Van't Veer MB, Bartelink H, van Leeuwen FE. Long-term cause-specific mortality of patients treated for Hodgkin's disease. J Clin Oncol. 2003; 21:3431–3439. PMID: 12885835.
Article2. van Leeuwen FE, Klokman WJ, Veer MB, et al. Long-term risk of second malignancy in survivors of Hodgkin's disease treated during adolescence or young adulthood. J Clin Oncol. 2000; 18:487–497. PMID: 10653864.
Article3. Sánchez-Aguilera A, Montalbán C, de la Cueva P, et al. Tumor microenvironment and mitotic checkpoint are key factors in the outcome of classic Hodgkin lymphoma. Blood. 2006; 108:662–668. PMID: 16551964.
Article4. Lau SK, Chu PG, Weiss LM. CD163: a specific marker of macrophages in paraffin-embedded tissue samples. Am J Clin Pathol. 2004; 122:794–801. PMID: 15491976.5. Jensen TO, Schmidt H, Møller HJ, et al. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J Clin Oncol. 2009; 27:3330–3337. PMID: 19528371.
Article6. Harris JA, Jain S, Ren Q, Zarineh A, Liu C, Ibrahim S. CD163 versus CD68 in tumor associated macrophages of classical Hodgkin lymphoma. Diagn Pathol. 2012; 7:12. PMID: 22289504.
Article7. Schaer DJ, Boretti FS, Hongegger A, et al. Molecular cloning and characterization of the mouse CD163 homologue, a highly glucocorticoid-inducible member of the scavenger receptor cysteine-rich family. Immunogenetics. 2001; 53:170–177. PMID: 11345593.
Article8. Schaer DJ, Boretti FS, Schoedon G, Schaffner A. Induction of the CD163-dependent haemoglobin uptake by macrophages as a novel anti-inflammatory action of glucocorticoids. Br J Haematol. 2002; 119:239–243. PMID: 12358930.
Article9. Galland F, Stefanova M, Lafage M, Birnbaum D. Localization of the 5' end of the MCF2 oncogene to human chromosome 15q15-q23. Cytogenet Cell Genet. 1992; 60:114–116. PMID: 1611909.10. Furge KA, Zhang YW, Vande Woude GF. Met receptor tyrosine kinase: enhanced signaling through adapter proteins. Oncogene. 2000; 19:5582–5589. PMID: 11114738.
Article11. Xu C, Plattel W, van den Berg A, et al. Expression of the c-Met oncogene by tumor cells predicts a favorable outcome in classical Hodgkin's lymphoma. Haematologica. 2012; 97:572–578. PMID: 22180430.
Article12. Kawano R, Ohshima K, Karube K, et al. Prognostic significance of hepatocyte growth factor and c-MET expression in patients with diffuse large B-cell lymphoma. Br J Haematol. 2004; 127:305–307. PMID: 15491290.
Article13. Bradwell AR, Carr-Smith HD, Mead GP, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001; 47:673–680. PMID: 11274017.
Article14. Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009; 23:215–224. PMID: 19020545.
Article15. Hutchison CA, Harding S, Hewins P, et al. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008; 3:1684–1690. PMID: 18945993.
Article16. Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002; 48:1437–1444. PMID: 12194920.17. Gottenberg JE, Aucouturier F, Goetz J, et al. Serum immunoglobulin free light chain assessment in rheumatoid arthritis and primary Sjogren's syndrome. Ann Rheum Dis. 2007; 66:23–27. PMID: 16569685.
Article18. van der Heijden M, Kraneveld A, Redegeld F. Free immunoglobulin light chains as target in the treatment of chronic inflammatory diseases. Eur J Pharmacol. 2006; 533:319–326. PMID: 16455071.
Article19. Swerdlow SH, Campo E, Harris NL, editors. WHO Classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC;2008.20. Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the committee on Hodgkin's disease staging classification. Cancer Res. 1971; 31:1860–1861. PMID: 5121694.21. Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med. 1998; 339:1506–1514. PMID: 9819449.22. Boleti E, Mead GM. ABVD for Hodgkin's lymphoma: full-dose chemotherapy without dose reductions or growth factors. Ann Oncol. 2007; 18:376–380. PMID: 17071938.
Article23. Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007; 25:579–586. PMID: 17242396.24. Yoon DH, Koh YW, Kang HJ, et al. CD68 and CD163 as prognostic factors for Korean patients with Hodgkin lymphoma. Eur J Haematol. 2012; 88:292–305. PMID: 22044760.
Article25. Hsi ED. Biologic features of Hodgkin lymphoma and the development of biologic prognostic factors in Hodgkin lymphoma: tumor and microenvironment. Leuk Lymphoma. 2008; 49:1668–1680. PMID: 18798102.
Article26. Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007; 26:373–400. PMID: 17717638.
Article27. Azambuja D, Natkunam Y, Biasoli I, et al. Lack of association of tumor-associated macrophages with clinical outcome in patients with classical Hodgkin's lymphoma. Ann Oncol. 2012; 23:736–742. PMID: 21602260.
Article28. Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010; 362:875–885. PMID: 20220182.
Article29. Tan KL, Scott DW, Hong F, et al. Tumor-associated macrophages predict inferior outcomes in classic Hodgkin lymphoma: a correlative study from the E2496 Intergroup trial. Blood. 2012; 120:3280–3287. PMID: 22948049.
Article30. Kamper P, Bendix K, Hamilton-Dutoit S, Honore B, d'Amore F. Tumor-infiltrating CD163-positive macrophages, clinicopathological parameters, and prognosis in classical Hodgkin lymphoma. J Clin Oncol. 2009; 27(Suppl):abst 8528.
Article31. Capello D, Gaidano G, Gallicchio M, et al. The tyrosine kinase receptor met and its ligand HGF are co-expressed and functionally active in HHV-8 positive primary effusion lymphoma. Leukemia. 2000; 14:285–291. PMID: 10673746.
Article32. Teofili L, Di Febo AL, Pierconti F, et al. Expression of the c-met proto-oncogene and its ligand, hepatocyte growth factor, in Hodgkin disease. Blood. 2001; 97:1063–1069. PMID: 11159538.
Article33. Bardelli A, Longati P, Albero D, et al. HGF receptor associates with the anti-apoptotic protein BAG-1 and prevents cell death. EMBO J. 1996; 15:6205–6212. PMID: 8947043.
Article34. Scott DW, Chan FC, Hong F, et al. Gene expression-based model using formalin-fixed paraffin-embedded biopsies predicts overall survival in advanced-stage classical Hodgkin lymphoma. J Clin Oncol. 2013; 31:692–700. PMID: 23182984.
Article35. Steidl C, Diepstra A, Lee T, et al. Gene expression profiling of microdissected Hodgkin Reed-Sternberg cells correlates with treatment outcome in classical Hodgkin lymphoma. Blood. 2012; 120:3530–3540. PMID: 22955918.
Article36. Nakopoulou L, Gakiopoulou H, Keramopoulos A, et al. c-met tyrosine kinase receptor expression is associated with abnormal beta-catenin expression and favourable prognostic factors in invasive breast carcinoma. Histopathology. 2000; 36:313–325. PMID: 10759945.37. Uddin S, Hussain AR, Ahmed M, et al. Inhibition of c-MET is a potential therapeutic strategy for treatment of diffuse large B-cell lymphoma. Lab Invest. 2010; 90:1346–1356. PMID: 20531293.
Article38. Lengyel E, Prechtel D, Resau JH, et al. C-Met overexpression in node-positive breast cancer identifies patients with poor clinical outcome independent of Her2/neu. Int J Cancer. 2005; 113:678–682. PMID: 15455388.
Article39. Thompson CA, Maurer MJ, Cerhan JR, et al. Elevated serum free light chains are associated with inferior event free and overall survival in Hodgkin lymphoma. Am J Hematol. 2011; 86:998–1000. PMID: 22006790.
Article40. De Filippi R, Russo F, Iaccarino G, et al. Abnormally elevated levels of serum free-immunoglobulin light chains are frequently found in classic Hodgkin lymphoma (cHL) and predict outcome of patients with early stage disease. Blood. 2009; 114(ASH Annual Meeting):abst 267.
Article41. De Filippi R, Morabito F, Corazzelli G, et al. Use of the cumulative amount of serum-free light chains (sFLC) at diagnosis and PET2 for the early identification of high risk of treatment failure in Hodgkin lymphoma (cHL). J Clin Oncol. 2012; 30(Suppl):abst 8083.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Classical Hodgkin lymphoma following follicular lymphoma: a case report
- C-met and E-cadherin Expression in Advanced Gastric Cancer
- Pathologic Characteristics and Differential Diagnosis of Hodgkin Lymphoma
- Diagnosis of lymphoma using bipedal lymphangiography
- Primary Non-Hodgkin T-Cell Lymphoma of the Esophagus