J Yeungnam Med Sci.
2023 Nov;40(Suppl):S113-S122. 10.12701/jyms.2023.00584.
Classical Hodgkin lymphoma following follicular lymphoma: a case report
- Affiliations
-
- 1Department of Pathology, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- KMID: 2548352
- DOI: http://doi.org/10.12701/jyms.2023.00584
Abstract
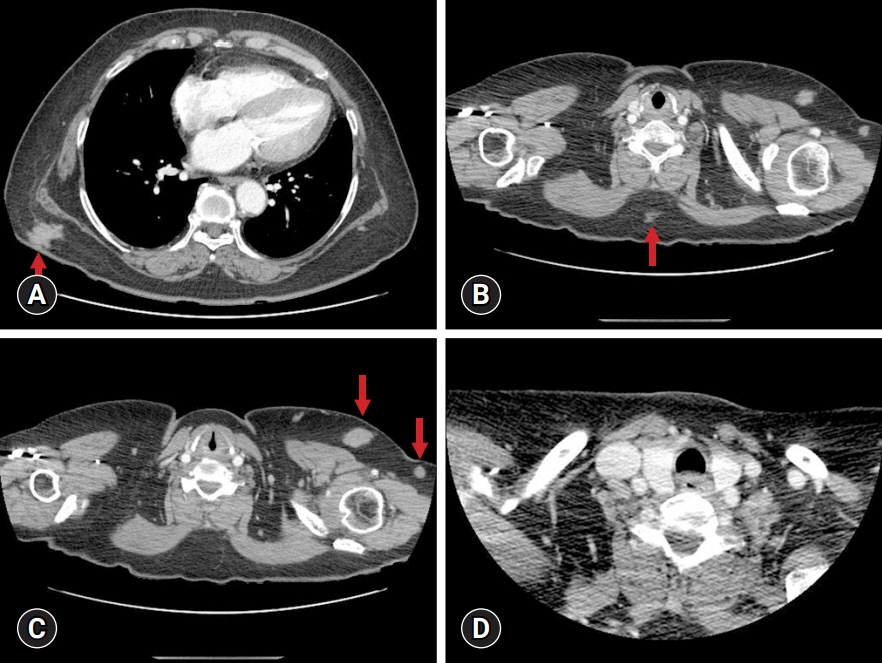
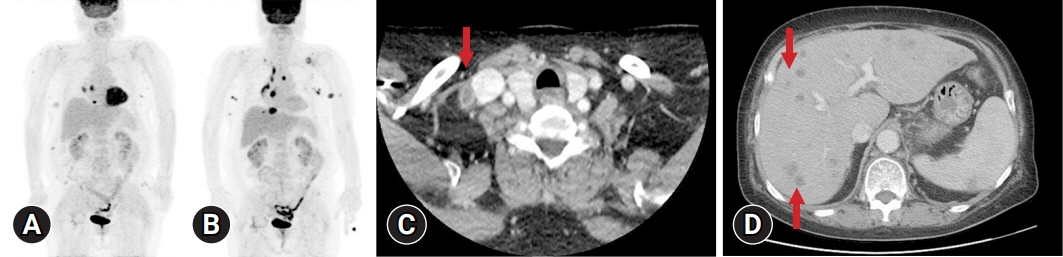
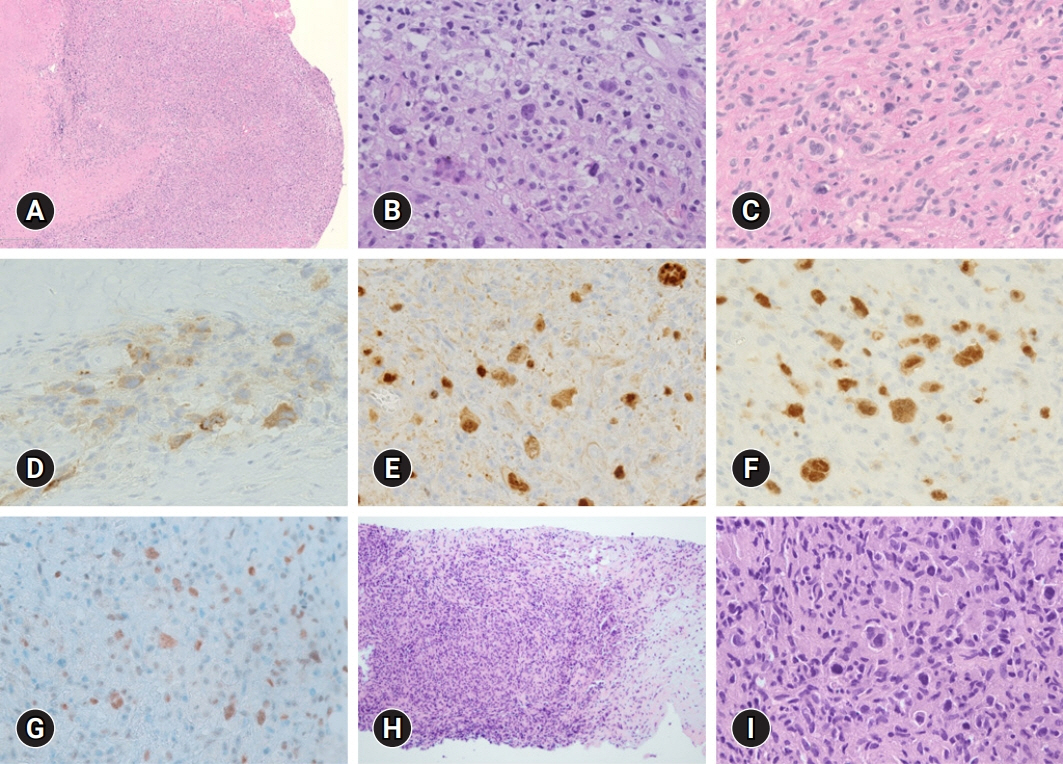
- The simultaneous, composite, or sequential occurrence of follicular lymphoma (FL) and classical Hodgkin lymphoma (HL), both of which originate from germinal center B-cell, is rare. Questions have been raised with regard to the type of tests that pathologists should perform when observing the presence of a “large-cell lymphoma” following an FL and what are the most critical pathological points for diagnosis. Here, we present a case of a classical HL following an FL after administering rituximab-bendamustine (R-Benda) chemotherapy. Furthermore, we also summarized the literature and compared this case with other HLs that followed FLs. A 55-year-old woman was diagnosed with a grade 3A FL of the breast and axillary lymph node masses. She completed six R-Benda chemotherapy cycles for stage IV FL. Twenty-three months after the diagnosis, follow-up image studies showed an increase in the size and number of the lesions. Biopsies of the neck lymph node and liver were performed, and the diagnosis was classical HL. Sequential or composite FL and HL may sometimes develop from the same clone because they share the same genetic alterations, such as B-cell lymphoma (Bcl)-2 or Bcl-6 translocation. When a large-cell lymphoma is found after the treatment of FL, classical HL should be considered a pathological differential diagnosis, and histological, immunohistochemical, or molecular investigations must be considered during the diagnostic process.
Keyword
Figure
Reference
-
References
1. Lossos IS, Gascoyne RD. Transformation of follicular lymphoma. Best Pract Res Clin Haematol. 2011; 24:147–63.
Article2. Jaffe ES, Zarate-Osorno A, Medeiros LJ. The interrelationship of Hodgkin’s disease and non-Hodgkin’s lymphomas: lessons learned from composite and sequential malignancies. Semin Diagn Pathol. 1992; 9:297–303.3. Huh J. Epidemiologic overview of malignant lymphoma. Korean J Hematol. 2012; 47:92–104.
Article4. Custer RP, Bernhard WG. The interrelationship of Hodgkin’s disease and other lymphatic tumors. Am J Med Sci. 1948; 216:625–42.
Article5. Carrato A, Filippa D, Koziner B. Hodgkin’s disease after treatment of non-Hodgkin’s lymphoma. Cancer. 1987; 60:887–96.
Article6. Lenner P, Roos G, Hedenus M, Lindh J. Simultaneous presentation of relapsing non-Hodgkin’s lymphoma and Hodgkin’s disease. Eur J Haematol. 1989; 42:315–6.
Article7. Gonzalez CL, Medeiros LJ, Jaffe ES. Composite lymphoma: a clinicopathologic analysis of nine patients with Hodgkin’s disease and B-cell non-Hodgkin’s lymphoma. Am J Clin Pathol. 1991; 96:81–9.8. Travis LB, Gonzalez CL, Hankey BF, Jaffe ES. Hodgkin’s disease following non-Hodgkin’s lymphoma. Cancer. 1992; 69:2337–42.
Article9. Zarate-Osorno A, Medeiros LJ, Kingma DW, Longo DL, Jaffe ES. Hodgkin’s disease following non-Hodgkin’s lymphoma: a clinicopathologic and immunophenotypic study of nine cases. Am J Surg Pathol. 1993; 17:123–32.10. LeBrun DP, Ngan BY, Weiss LM, Huie P, Warnke RA, Cleary ML. The bcl-2 oncogene in Hodgkin’s disease arising in the setting of follicular non-Hodgkin’s lymphoma. Blood. 1994; 83:223–30.
Article11. Hirose Y, Iwabuchi K, Shimizu S, Sasaki K, Nojima T, Takiguchi T. Nodal EBV-positive Hodgkin’s disease following extranodal EBV negative non-Hodgkin’s lymphoma of B-cell lineage. Eur J Haematol. 1996; 57:103–6.12. Thirumala S, Esposito M, Fuchs A. An unusual variant of composite lymphoma: a short case report and review of the literature. Arch Pathol Lab Med. 2000; 124:1376–8.13. Copur MS, Ledakis P, Novinski D, Fu K, Hutchins M, Frankforter S, et al. An unusual case of composite lymphoma involving chronic lymphocytic leukemia follicular lymphoma and Hodgkin disease. Leuk Lymphoma. 2004; 45:1071–6.
Article14. Nakamura N, Ohshima K, Abe M, Osamura Y. Demonstration of chimeric DNA of bcl-2 and immunoglobulin heavy chain in follicular lymphoma and subsequent Hodgkin lymphoma from the same patient. J Clin Exp Hematop. 2007; 47:9–13.
Article15. Yoshida M, Ichikawa A, Miyoshi H, Takeuchi M, Kimura Y, Nino D, et al. High frequency of t(14;18) in Hodgkin’s lymphoma associated with follicular lymphoma. Pathol Int. 2012; 62:518–24.
Article16. Wang XJ, Griffin GK, Yenamandra A, Wheeler FC, Ligon AH, Nandedka MA, et al. Transformation of follicular lymphoma into classical Hodgkin lymphoma showing t(14;18). Hematopathology. 2016; 1:23–33.17. Tennese A, Skrabek PJ, Nasr MR, Sekiguchi DR, Morales C, Brown TC, et al. Four lymphomas in 1 patient: a unique case of triple composite non-Hodgkin lymphoma followed by classical Hodgkin lymphoma. Int J Surg Pathol. 2017; 25:276–80.
Article18. Kim M, Hwang HS, Cho H, Yoon DH, Suh C, Park CS, et al. Upward trend in follicular lymphoma among the Korean population: 10-year experience at a large tertiary institution. J Pathol Transl Med. 2021; 55:330–7.
Article19. Küppers R, Sousa AB, Baur AS, Strickler JG, Rajewsky K, Hansmann ML. Common germinal-center B-cell origin of the malignant cells in two composite lymphomas, involving classical Hodgkin’s disease and either follicular lymphoma or B-CLL. Mol Med. 2001; 7:285–92.
Article20. Trecourt A, Mauduit C, Szablewski V, Fontaine J, Balme B, Donzel M, et al. Plasticity of mature B cells between follicular and classic Hodgkin lymphomas: a series of 22 cases expanding the spectrum of transdifferentiation. Am J Surg Pathol. 2022; 46:58–70.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Composite follicular lymphoma and classic Hodgkin lymphoma
- Pathologic Characteristics and Differential Diagnosis of Hodgkin Lymphoma
- A Case of Recurred Follicular Lymphoma in Sublingual Gland after Complete Remission
- In Situ Follicular Lymphoma Developed after Hodgkin Lymphoma
- Epstein-Barr Virus Positive Follicular Lymphoma of Lymph Node