Blood Res.
2014 Sep;49(3):154-161. 10.5045/br.2014.49.3.154.
Development of NK cell expansion methods using feeder cells from human myelogenous leukemia cell line
- Affiliations
-
- 1Department of Biology Education, College of Education, Chungbuk National University, Cheongju, Korea. chemokine@cbnu.ac.kr
- KMID: 2172786
- DOI: http://doi.org/10.5045/br.2014.49.3.154
Abstract
- BACKGROUND
Natural killer (NK) cells constantly survey surrounding tissues and remove newly generated cancer cells, independent of cancer antigen recognition. Although there have been a number of attempts to apply NK cells for cancer therapy, clinical application has been somewhat limited because of the difficulty in preparing a sufficient number of NK cells. Therefore, ex vivo NK cell expansion is one of the important steps for developing NK cell therapeutics.
METHODS
CD3+ depleted lymphocytes were cocultured with IL-2 and with feeder cells (peripheral blood mononuclear cells [PBMCs], K562, and Jurkat) for 15 days. Expanded NK cells were tested for cytotoxicity against cancer cell lines.
RESULTS
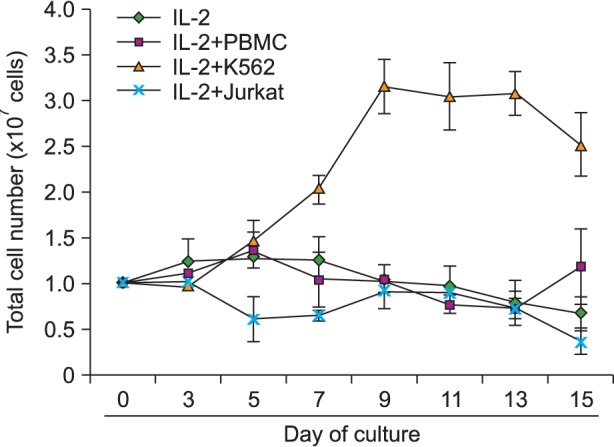
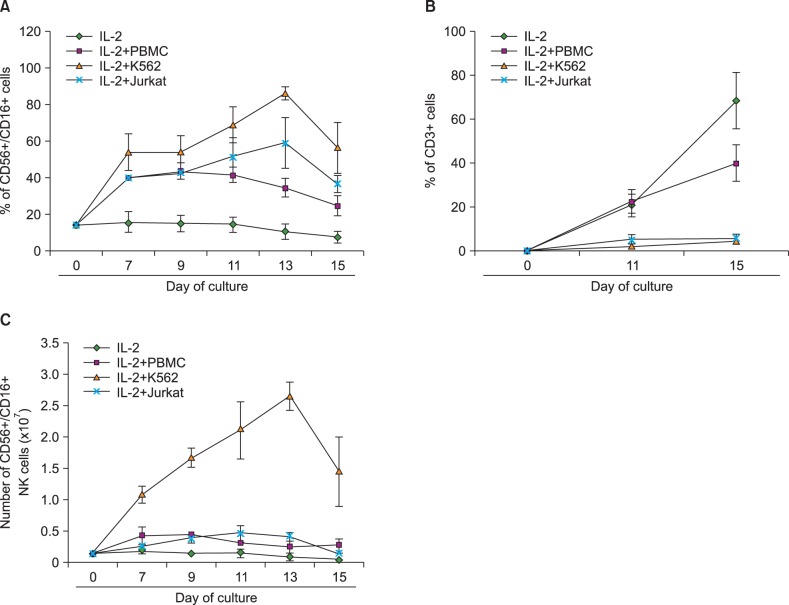
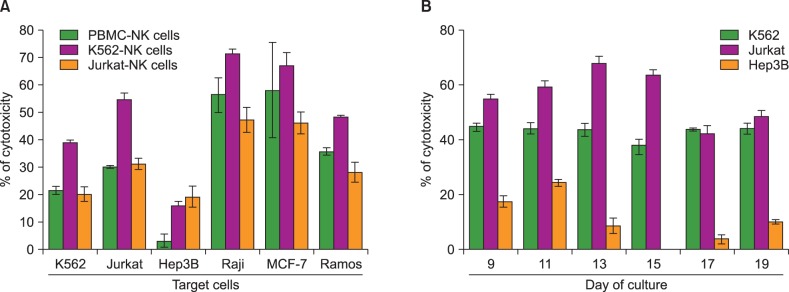
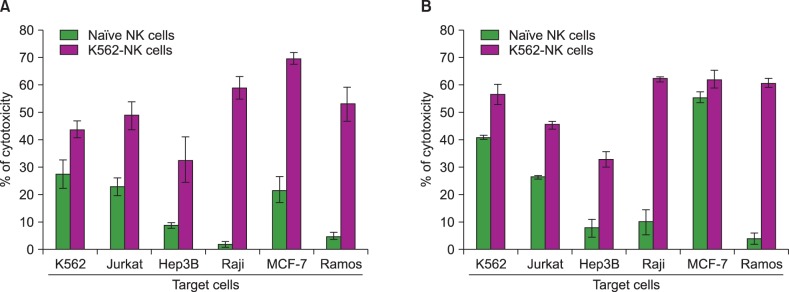
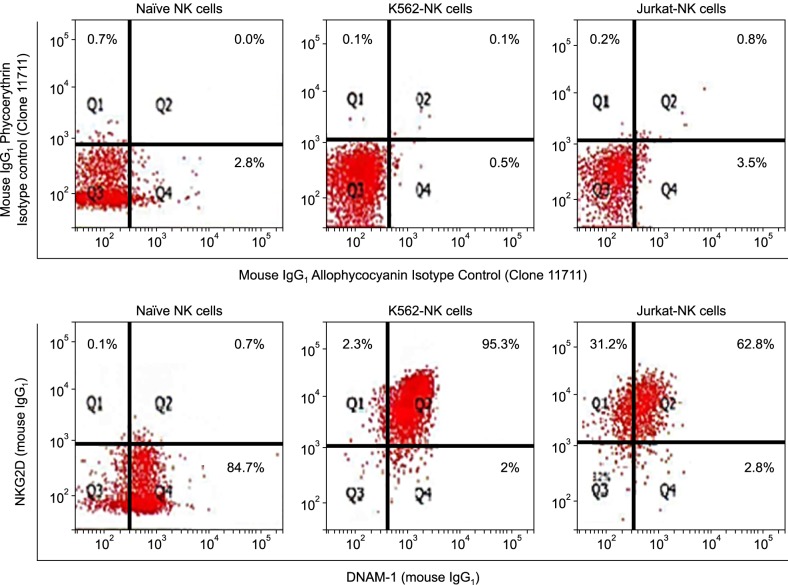
We compared feeder activities of three different cells-PBMC, K562, and Jurkat. K562 expanded NK cells by almost 20 fold and also showed powerful cytotoxic activity against cancer cells. K562-NK cells remarkably expressed the NK cell activation receptors, NKG2D, and DNAM-1. K562-NK cells exhibited more than two-fold production of cytotoxic granules compared with Jurkat-NK cells, producing more perforin and granzyme B than naive NK cells.
CONCLUSION
Our findings suggest that K562 are more efficient feeder cells than Jurkat or PBMCs. K562 feeder cells expanded NK cells by almost 20 fold and showed powerful cytotoxic activity against cancer cells. We herein propose an intriguing approach for a design of NK cell expansion.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Way to go to exploit NK cells' versatile talents for cancer immunotherapy
Duck Cho
Blood Res. 2014;49(3):139-140. doi: 10.5045/br.2014.49.3.139.
Reference
-
1. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008; 9:503–510. PMID: 18425107.
Article2. Perussia B, Ramoni C, Anegon I, Cuturi MC, Faust J, Trinchieri G. Preferential proliferation of natural killer cells among peripheral blood mononuclear cells cocultured with B lymphoblastoid cell lines. Nat Immun Cell Growth Regul. 1987; 6:171–188. PMID: 2960890.3. Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol. 2002; 2:957–964. PMID: 12461568.
Article4. Moretta A, Bottino C, Vitale M, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001; 19:197–223. PMID: 11244035.
Article5. Houchins JP, Yabe T, McSherry C, Bach FH. DNA sequence analysis of NKG2, a family of related cDNA clones encoding type II integral membrane proteins on human natural killer cells. J Exp Med. 1991; 173:1017–1020. PMID: 2007850.
Article6. Tahara-Hanaoka S, Shibuya K, Onoda Y, et al. Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112). Int Immunol. 2004; 16:533–538. PMID: 15039383.
Article7. Zhang C, Zhang J, Niu J, Zhou Z, Zhang J, Tian Z. Interleukin-12 improves cytotoxicity of natural killer cells via upregulated expression of NKG2D. Hum Immunol. 2008; 69:490–500. PMID: 18619507.
Article8. Armeanu S, Bitzer M, Lauer UM, et al. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res. 2005; 65:6321–6329. PMID: 16024634.
Article9. Zhang C, Zhang J, Sun R, Feng J, Wei H, Tian Z. Opposing effect of IFNgamma and IFNalpha on expression of NKG2 receptors: negative regulation of IFNgamma on NK cells. Int Immunopharmacol. 2005; 5:1057–1067. PMID: 15829421.10. Fuchshuber PR, Lotzova E. Feeder cells enhance oncolytic and proliferative activity of long-term human bone marrow interleukin-2 cultures and induce different lymphocyte subsets. Cancer Immunol Immunother. 1991; 33:15–20. PMID: 2021955.
Article11. Rabinowich H, Sedlmayr P, Herberman RB, Whiteside TL. Increased proliferation, lytic activity, and purity of human natural killer cells cocultured with mitogen-activated feeder cells. Cell Immunol. 1991; 135:454–470. PMID: 1709827.
Article12. Boissel L, Tuncer HH, Betancur M, Wolfberg A, Klingemann H. Umbilical cord mesenchymal stem cells increase expansion of cord blood natural killer cells. Biol Blood Marrow Transplant. 2008; 14:1031–1038. PMID: 18721766.
Article13. Kabelitz D. Human cytotoxic andclonal specificity of CD8+CD16-(Leu2+Leu11-) and CD16+CD3-(Leu11+Leu4-) cytotoxic lymphocyte precursors activated byalloantigen or K562 stimulator cells. Cell Immunol. 1989; 121:298–305. PMID: 2525425.14. Koepsell SA, Miller JS, McKenna DH Jr. Natural killer cells: a review of manufacturing and clinical utility. Transfusion. 2013; 53:404–410. PMID: 22670662.
Article15. Lapteva N, Durett AG, Sun J, et al. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy. 2012; 14:1131–1143. PMID: 22900959.
Article16. Harada H, Saijo K, Watanabe S, et al. Selective expansion of human natural killer cells from peripheral blood mononuclear cells by the cell line, HFWT. Jpn J Cancer Res. 2002; 93:313–319. PMID: 11927014.
Article17. Warren HS, Skipsey LJ. Phenotypic analysis of a resting subpopulation of human peripheral blood NK cells: the FcR gamma III (CD16) molecule and NK cell differentiation. Immunology. 1991; 72:150–157. PMID: 1825480.18. Miller JS, Oelkers S, Verfaillie C, McGlave P. Role of monocytes in the expansion of human activated natural killer cells. Blood. 1992; 80:2221–2229. PMID: 1421393.
Article19. Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005; 106:376–383. PMID: 15755898.
Article20. Denman CJ, Senyukov VV, Somanchi SS, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One. 2012; 7:e30264. PMID: 22279576.
Article21. Hart MK, Kornbluth J, Main EK, Spear BT, Taylor J, Wilson DB. Lymphocyte function-associated antigen 1 (LFA-1) and natural killer (NK) cell activity: LFA-1 is not necessary for all killer: target cell interactions. Cell Immunol. 1987; 109:306–317. PMID: 3311386.22. Bae DS, Hwang YK, Lee JK. Importance of NKG2D-NKG2D ligands interaction for cytolytic activity of natural killer cell. Cell Immunol. 2012; 276:122–127. PMID: 22613008.
Article23. Lim O, Lee Y, Chung H, et al. GMP-compliant, large-scale expanded allogeneic natural killer cells have potent cytolytic activity against cancer cells in vitro and in vivo. PLoS One. 2013; 8:e53611. PMID: 23326467.
Article24. Ahn YO, Kim S, Kim TM, Song EY, Park MH, Heo DS. Irradiated and activated autologous PBMCs induce expansion of highly cytotoxic human NK cells in vitro. J Immunother. 2013; 36:373–381. PMID: 23924789.
Article25. Guerra N, Tan YX, Joncker NT, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008; 28:571–580. PMID: 18394936.
Article26. Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003; 3:781–790. PMID: 14523385.
Article27. Marusina AI, Burgess SJ, Pathmanathan I, Borrego F, Coligan JE. Regulation of human DAP10 gene expression in NK and T cells by Ap-1 transcription factors. J Immunol. 2008; 180:409–417. PMID: 18097042.28. Castriconi R, Dondero A, Corrias MV, et al. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: critical role of DNAX accessory molecule-1-poliovirus receptor interaction. Cancer Res. 2004; 64:9180–9184. PMID: 15604290.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expansion and Activation of Natural Killer Cells for Cancer Immunotherapy
- The Use of Canine Bone-Marrow Stromal Cell Line, DO64, as Feeder Cells for The Efficient Cloning of Human B Lymphoblastoid Cell Lines ( B-LCLs )
- Development of peripheral T-cell lymphoma in the course of chronic myelogenous leukemia
- Engineering Biomaterials for Feeder-Free Maintenance of Human Pluripotent Stem Cells
- Selective Expansion of Natural Killer Cells from Peripheral Blood Mononuclear Cells by K562 Cell Line and IL-2