Korean J Lab Med.
2009 Apr;29(2):89-96. 10.3343/kjlm.2009.29.2.89.
Expansion and Activation of Natural Killer Cells for Cancer Immunotherapy
- Affiliations
-
- 1Department of Oncology, St. Jude Children's Research Hospital, Memphis, TN, USA. dario.campana@stjude.org
- 2Department of Pathology, St. Jude Children's Research Hospital, Memphis, TN, USA.
- KMID: 854962
- DOI: http://doi.org/10.3343/kjlm.2009.29.2.89
Abstract
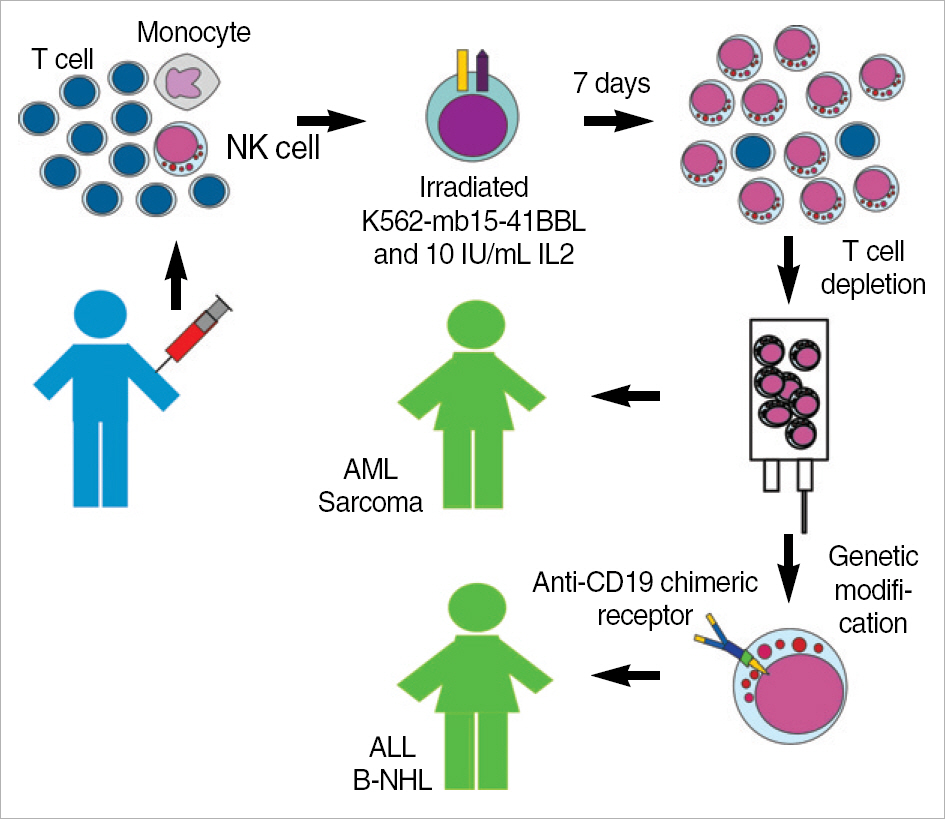
- Natural killer (NK) cells can kill a wide range of cancer cells and are a promising tool for cell therapy of cancer. NK cells cytotoxicity is regulated by a balance between stimulatory and inhibitory signals. Interleukin-2 is known to increase NK cell cytotoxicity. Although many cytokines have been studied in efforts to induce durable NK cell expansions, most reports indicate a rather modest effect and the requirement for additional stimuli. We found that contact with the K562 myeloid leukemia cell line, genetically modified to express a membrane-bound form of interleukin-15 and the ligand for the costimulatory molecule 4-1BB, induced vigorous expansion of NK cells from peripheral blood. Based on these findings, we developed a method for large-scale clinical-grade expansion of NK cells. This method is currently used to expand allogeneic NK cells for infusion in patients with leukemia and solid tumors. We here summarize methods for expansion and activation of NK cells from human peripheral blood mononuclear cells as well as clinical-scale methods to produce NK cells for immunotherapy under Current Good Manufacturing Practices (cGMP) conditions.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Way to go to exploit NK cells' versatile talents for cancer immunotherapy
Duck Cho
Blood Res. 2014;49(3):139-140. doi: 10.5045/br.2014.49.3.139.
Reference
-
1.Galy A., Travis M., Cen D., Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995. 3:459–73.
Article2.Farag SS., Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2006. 20:123–37.
Article3.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005. 23:225–74.
Article4.Caligiuri MA. Human natural killer cells. Blood. 2008. 112:461–9.
Article5.Cooper MA., Fehniger TA., Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001. 22:633–40.
Article6.Fehniger TA., Cooper MA., Nuovo GJ., Cella M., Facchetti F., Colonna M, et al. CD56 bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003. 101:3052–7.7.Lundqvist A., Abrams SI., Schrump DS., Alvarez G., Suffredini D., Berg M, et al. Bortezomib and depsipeptide sensitize tumors to tumor necrosis factor-related apoptosis-inducing ligand: a novel method to potentiate natural killer cell tumor cytotoxicity. Cancer Res. 2006. 66:7317–25.
Article8.Srivastava S., Lundqvist A., Childs RW. Natural killer cell immunotherapy for cancer: a new hope. Cytotherapy. 2008. 10:775–83.
Article9.Moretta L., Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004. 23:255–9.
Article10.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008. 9:495–502.
Article11.Ljunggren HG., Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990. 11:237–44.
Article12.Yokoyama WM., Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006. 214:143–54.
Article13.Terme M., Ullrich E., Delahaye NF., Chaput N., Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol. 2008. 9:486–94.
Article14.Trinchieri G., Matsumoto-Kobayashi M., Clark SC., Seehra J., London L., Perussia B. Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med. 1984. 160:1147–69.
Article15.Phillips JH., Lanier LL. Dissection of the lymphokine-activated killer phenomenon. Relative contribution of peripheral blood natural killer cells and T lymphocytes to cytolysis. J Exp Med. 1986. 164:814–25.
Article16.London L., Perussia B., Trinchieri G. Induction of proliferation in vitro of resting human natural killer cells: IL 2 induces into cell cycle most peripheral blood NK cells, but only a minor subset of low density T cells. J Immunol. 1986. 137:3845–54.17.Lanier LL., Buck DW., Rhodes L., Ding A., Evans E., Barney C, et al. Interleukin 2 activation of natural killer cells rapidly induces the expression and phosphorylation of the Leu-23 activation antigen. J Exp Med. 1988. 167:1572–85.
Article18.Robertson MJ., Manley TJ., Donahue C., Levine H., Ritz J. Costimulatory signals are required for optimal proliferation of human natural killer cells. J Immunol. 1993. 150:1705–14.19.Imai C., Iwamoto S., Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005. 106:376–83.
Article20.Alici E., Sutlu T., Bjorkstrand B., Gilljam M., Stellan B., Nahi H, et al. Autologous antitumor activity by NK cells expanded from myeloma patients using GMP-compliant components. Blood. 2008. 111:3155–62.
Article21.Carlens S., Gilljam M., Chambers BJ., Aschan J., Guven H., Ljunggren HG, et al. A new method for in vitro expansion of cytotoxic human CD3-CD56+ natural killer cells. Hum Immunol. 2001. 62:1092–8.
Article22.Rosenberg SA., Lotze MT., Muul LM., Leitman S., Chang AE., Ettinghausen SE, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985. 313:1485–92.
Article23.Miller JS., Oelkers S., Verfaillie C., McGlave P. Role of monocytes in the expansion of human activated natural killer cells. Blood. 1992. 80:2221–9.
Article24.Robertson MJ., Cameron C., Lazo S., Cochran KJ., Voss SD., Ritz J. Costimulation of human natural killer cell proliferation: role of accessory cytokines and cell contact-dependent signals. Nat Immun. 1996-1997. 15:213–26.25.Rabinowich H., Sedlmayr P., Herberman RB., Whiteside TL. Increased proliferation, lytic activity, and purity of human natural killer cells cocultured with mitogen-activated feeder cells. Cell Immunol. 1991. 135:454–70.
Article26.Igarashi T., Wynberg J., Srinivasan R., Becknell B., McCoy JP Jr., Takahashi Y, et al. Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood. 2004. 104:170–7.
Article27.Perussia B., Ramoni C., Anegon I., Cuturi MC., Faust J., Trinchieri G. Preferential proliferation of natural killer cells among peripheral blood mononuclear cells cocultured with B lymphoblastoid cell lines. Nat Immun Cell Growth Regul. 1987. 6:171–88.28.Harada H., Saijo K., Watanabe S., Tsuboi K., Nose T., Ishiwata I, et al. Selective expansion of human natural killer cells from peripheral blood mononuclear cells by the cell line, HFWT. Jpn J Cancer Res. 2002. 93:313–9.
Article29.Luhm J., Brand JM., Koritke P., Hoppner M., Kirchner H., Frohn C. Large-scale generation of natural killer lymphocytes for clinical application. J Hematother Stem Cell Res. 2002. 11:651–7.
Article30.Condiotti R., Zakai YB., Barak V., Nagler A. Ex vivo expansion of CD56+ cytotoxic cells from human umbilical cord blood. Exp Hematol. 2001. 29:104–13.
Article31.Boissel L., Tuncer HH., Betancur M., Wolfberg A., Klingemann H. Umbilical cord mesenchymal stem cells increase expansion of cord blood natural killer cells. Biol Blood Marrow Transplant. 2008. 14:1031–8.
Article32.Phillips JH., Lanier LL. A model for the differentiation of human natural killer cells. Studies on the in vitro activation of Leu-11+ granular lymphocytes with a natural killer-sensitive tumor cell, K562. J Exp Med. 1985. 161:1464–82.
Article33.Melero I., Johnston JV., Shufford WW., Mittler RS., Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998. 190:167–72.
Article34.Carson WE., Fehniger TA., Haldar S., Eckhert K., Lindemann MJ., Lai CF, et al. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest. 1997. 99:937–43.
Article35.Cooper MA., Bush JE., Fehniger TA., VanDeusen JB., Waite RE., Liu Y, et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002. 100:3633–8.
Article36.Fehniger TA., Caligiuri MA. Ontogeny and expansion of human natural killer cells: clinical implications. Int Rev Immunol. 2001. 20:503–34.
Article37.Wu J., Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003. 90:127–56.
Article38.Musso T., Calosso L., Zucca M., Millesimo M., Ravarino D., Giovarelli M, et al. Human monocytes constitutively express membrane-bound, biologically active, and interferon-gamma-upregulated interleukin-15. Blood. 1999. 93:3531–9.39.Dubois S., Mariner J., Waldmann TA., Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002. 17:537–47.40.Koka R., Burkett P., Chien M., Chai S., Boone DL., Ma A. Cutting edge: murine dendritic cells require IL-15R alpha to prime NK cells. J Immunol. 2004. 173:3594–8.41.Burkett PR., Koka R., Chien M., Chai S., Boone DL., Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004. 200:825–34.42.Kobayashi H., Dubois S., Sato N., Sabzevari H., Sakai Y., Waldmann TA, et al. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood. 2005. 105:721–7.
Article43.Fujisaki H., Kakuda H., Shimasaki N., Imai C., Ma J., Lockey T, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009. In press.
Article44.Leung W., Iyengar R., Leimig T., Holladay MS., Houston J., Handgretinger R. Phenotype and function of human natural killer cells purified by using a clinical-scale immunomagnetic method. Cancer Immunol Immunother. 2005. 54:389–94.
Article45.Meehan KR., Wu J., Webber SM., Barber A., Szczepiorkowski ZM., Sentman C. Development of a clinical model for ex vivo expansion of multiple populations of effector cells for adoptive cellular therapy. Cytotherapy. 2008. 10:30–7.
Article46.Miller JS., Soignier Y., Panoskaltsis-Mortari A., McNearney SA., Yun GH., Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005. 105:3051–7.
Article47.Klingemann HG., Martinson J. Ex vivo expansion of natural killer cells for clinical applications. Cytotherapy. 2004. 6:15–22.
Article48.McKenna DH Jr., Sumstad D., Bostrom N., Kadidlo DM., Fautsch S., McNearney S, et al. Good manufacturing practices production of natural killer cells for immunotherapy: a six-year single-institution experience. Transfusion. 2007. 47:520–8.
Article49.Torelli GF., Guarini A., Maggio R., Alfieri C., Vitale A., Foa R. Expansion of natural killer cells with lytic activity against autologous blasts from adult and pediatric acute lymphoid leukemia patients in complete hematologic remission. Haematologica. 2005. 90:785–92.50.Tam YK., Martinson JA., Doligosa K., Klingemann HG. Ex vivo expansion of the highly cytotoxic human natural killer-92 cell-line under current good manufacturing practice conditions for clinical adoptive cellular immunotherapy. Cytotherapy. 2003. 5:259–72.51.Ruggeri L., Capanni M., Urbani E., Perruccio K., Shlomchik WD., Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002. 295:2097–100.
Article52.Ruggeri L., Capanni M., Casucci M., Volpi I., Tosti A., Perruccio K, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999. 94:333–9.
Article53.Giebel S., Locatelli F., Lamparelli T., Velardi A., Davies S., Frumento G, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003. 102:814–9.
Article54.Leung W., Iyengar R., Turner V., Lang P., Bader P., Conn P, et al. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004. 172:644–50.
Article55.Rooney CM., Smith CA., Ng CY., Loftin SK., Sixbey JW., Gan Y, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998. 92:1549–55.
Article56.Wagner HJ., Bollard CM., Vigouroux S., Huls MH., Anderson R., Prentice HG, et al. A strategy for treatment of Epstein-Barr virus-positive Hodgkin's disease by targeting interleukin 12 to the tumor environment using tumor antigen-specific T cells. Cancer Gene Ther. 2004. 11:81–91.
Article57.Comoli P., De Palma R., Siena S., Nocera A., Basso S., Del Galdo F, et al. Adoptive transfer of allogeneic Epstein-Barr virus (EBV)-specific cytotoxic T cells with in vitro antitumor activity boosts LMP2-specific immune response in a patient with EBV-related nasopharyngeal carcinoma. Ann Oncol. 2004. 15:113–7.58.Klein G., Klein E. Surveillance against tumors–is it mainly immunological? Immunol Lett. 2005. 100:29–33.
Article59.Main EK., Lampson LA., Hart MK., Kornbluth J., Wilson DB. Human neuroblastoma cell lines are susceptible to lysis by natural killer cells but not by cytotoxic T lymphocytes. J Immunol. 1985. 135:242–6.60.Raffaghello L., Prigione I., Airoldi I., Camoriano M., Morandi F., Bocca P, et al. Mechanisms of immune evasion of human neuroblastoma. Cancer Letters. 2005. 228:155–61.
Article61.Sadelain M., Riviere I., Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003. 3:35–45.
Article62.Imai C., Campana D. Genetic modification of T cells for cancer therapy. J Biol Regul Homeost Agents. 2004. 18:62–71.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Natural Killer Cell and Cancer Immunotherapy
- Natural Killer Cells and Thyroid Diseases
- Ex vivo Expansion of Human Natural Killer Cells from Blood Retained in a Disposable Platelet Apheresis Set
- Molecular checkpoints controlling natural killer cell activation and their modulation for cancer immunotherapy
- A Case of Management for Advanced Hepatocellular Carcinoma with Extrahepatic Metastasis by Autologous Natural Killer Cells Combined with Immune Checkpoint Inhibitor