Chonnam Med J.
2009 Apr;45(1):38-46. 10.4068/cmj.2009.45.1.38.
Effects of micron-Opioid Agonist on ATP-sensitive Potassium Channel Activity in Isolated Ventricular Cardiomyocytes
- Affiliations
-
- 1Department of Pharmacology, Chonnam National University Medical School, Chonnam National University Research Institute of Medical Sciences, and The Brain Korea 21 Project, Center for Biomedical Human Resources at Chonnam National University, Gwangju, Kore
- KMID: 2172282
- DOI: http://doi.org/10.4068/cmj.2009.45.1.38
Abstract
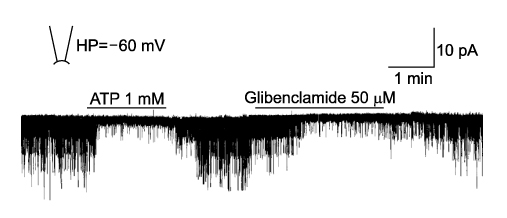
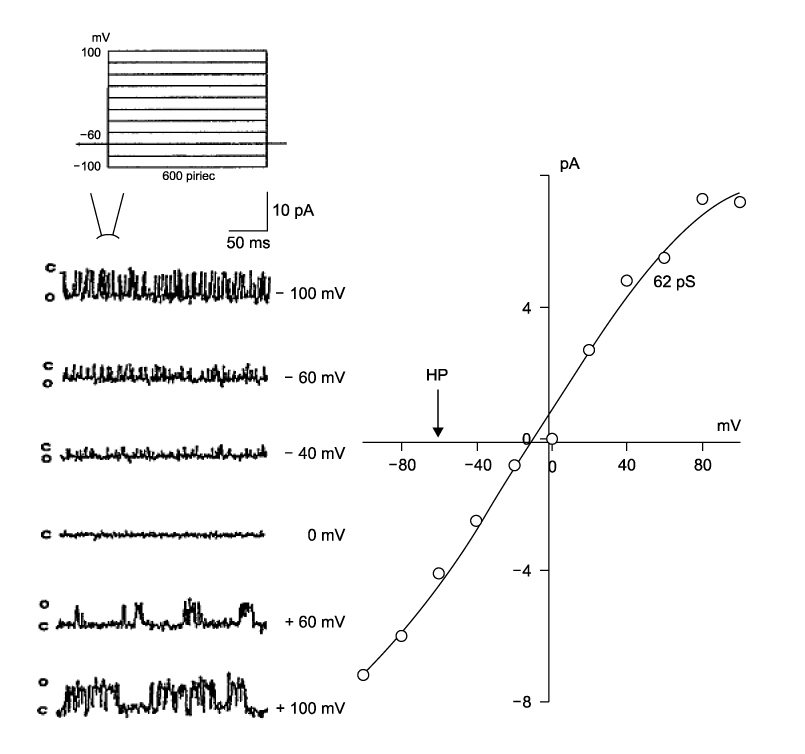
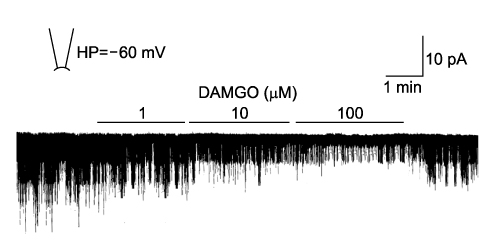
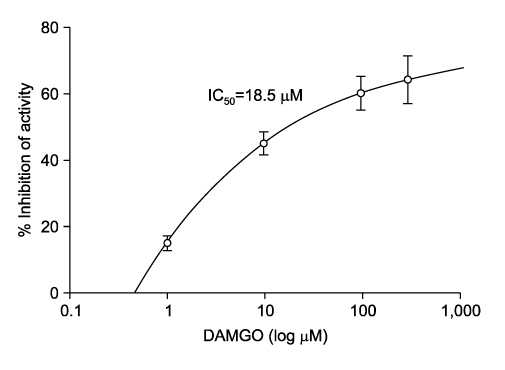
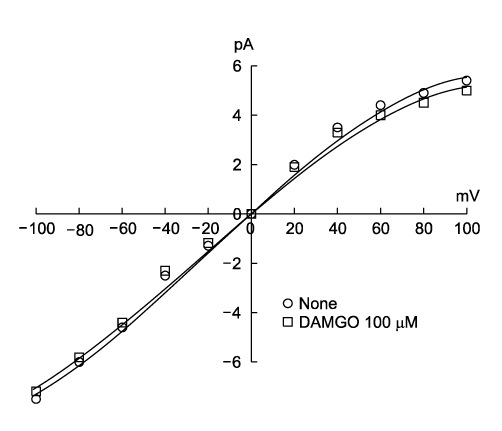
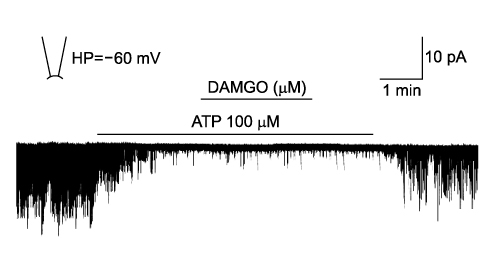
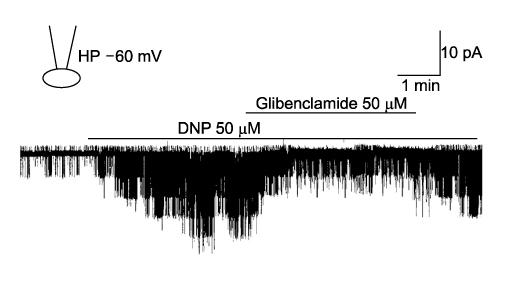
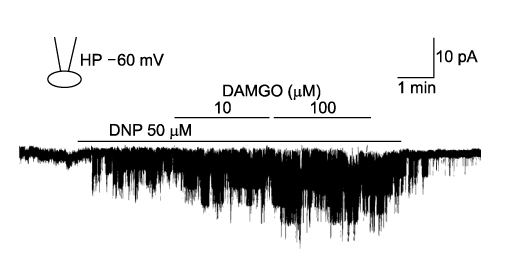
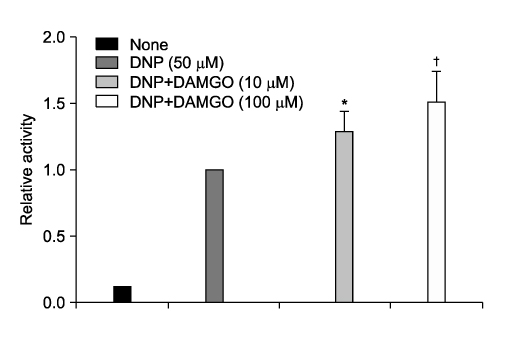
- It is well known that opioid agonists are released from the myocardium during hypoxia or ischemia, and that ATP-sensitive potassium channels (K(ATP) channels) exist in the cardiac cell membrane and function as a cardioprotector preventing the myocardium from ischemic damage. In the present study, therefore, to determine whether opioid agonists are involved in the regulation of ATP-sensitive potassium channel activities, effects of the micron-opioid agonist DAMGO were examined on K(ATP) channel activities by using excised inside-out and cell-attached patch clamp techniques in enzymatically (collagenase and protease) isolated mouse ventricular cardiac myocytes. In the excised inside-out patches, DAMGO (1~300 micron mol/L) inhibited K(ATP) channel activities in a dose-dependent manner. K(ATP) channel activity, which had been attenuated by the addition of ATP (100 micron mol/L) to the internal solution, was not reactivated by DAMGO. The fashion of the single-channel inhibition by DAMGO was that both channel opening frequency and mean open-time were decreased, but the amplitudes of single channel currents and channel conductances were not altered. The half-maximal inhibition concentration (IC50) for DAMGO was 18 micron mol/L. In the cell- attached patch configuration, however, DAMGO (1~300 micron mol/L) increased dinitrophenol (50 micron mol/L)- induced K(ATP) channel activities. It was inferred that the micron-opioid agonist is involved in the regulation of ATP-sensitive potassium channel activity in cardiac myocardium, agonizing (through internal target) or antagonizing (through external target) the inhibitory action of ATP in a competitive manner, thereby attenuating or enhancing the channel openings.
MeSH Terms
-
Adenosine Triphosphate
Animals
Anoxia
Cell Membrane
Enkephalin, Ala(2)-MePhe(4)-Gly(5)-
Felodipine
Ischemia
KATP Channels
Mice
Myocardium
Myocytes, Cardiac
Patch-Clamp Techniques
Potassium
Potassium Channels
Adenosine Triphosphate
Enkephalin, Ala(2)-MePhe(4)-Gly(5)-
Felodipine
KATP Channels
Potassium
Potassium Channels
Figure
Reference
-
1. Oldroyd KG, Harvey K, Gray CE, Beastall GH, Cobbe SM. Beta endorphin release in patients after spontaneous and provoked acute myocardial ischaemia. Br Heart J. 1992. 67:230–235.
Article2. Paradis P, Dumont M, Belichard P, Rouleau JL, Lemaire S, Brakier-Gingras L. Increased preproenkephalin A gene expression in the rat heart after induction of a myocardial infarction. Biochem Cell Biol. 1992. 70:593–598.
Article3. Ytrehus K, Liu Y, Downey JM. Preconditioning protects ischemic rabbit heart by protein kinase C activation. Am J Physiol. 1994. 266:H1145–H1152.
Article4. Weiss J, Lamp ST. Cardiac ATP-sensitive K+ channels. Evidence for preferential regulation by glycolysis. J Gen Physiol. 1989. 94:911–935.
Article5. Noma A, Takano M. The ATP-sensitive K+ channel. JPN J Physiol. 1991. 41:177–187.
Article6. Escande D, Cavero I. Escande D, Standen N, editors. Potassium channel openers in the heart. K+ Channels in cardiovascular medicine. 1993. Paris: Springer-Verlag;225–244.7. Maslov LN, Lishmanov YB. Change in opioid peptide level in the heart and blood plasma during acute myocardial ischemia complicated by ventricular fibrillation. Clin Exp Pharmacol Physiol. 1995. 22:812–816.
Article8. Wong TM, Lee AY, Tai KK. Effects of drugs interacting with opioid receptors during normal perfusion or ischemia and reperfusion in the isolated rat heart. an attempt to identify cardiac opioid receptor subtype(s) involved in arrhythmogenesis. J Mol Cell Cardiol. 1990. 22:1167–1175.
Article9. Alarcón S, Hernandez J, Laorden ML. Diazoxide blocks the morphine induced lengthening of action potential duration on guinea-pig papillary muscle. Gen Pharmacol. 1995. 26:589–592.
Article10. Xiao GS, Zhou JJ, Cheung YF, Li GR, Wong TM. Effects of U50,488H on transient outward and ultra-rapid delayed rectifier K+ currents in young human atrial myocytes. Euro J Pharmacol. 2003. 473:97–103.
Article11. Cao Z, Liu L, Van Winkle DM. Activation of δ- and kappa-opioid receptors by opioid peptides protects cardiomyocytes via KATP channels. Am J Physiol. 2003. 285:H1032–H1039.12. Piao LH, Kim JH. Role of plasmalogen-derived lysolipids on the pathophysiology of myocardial ischemia. Chonnam Medical J. 2007. 43:168–176.13. Spruce AE, Standen NB, Stanfield PR. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature. 1985. 316:736–738.
Article14. Xu X, Lee KS. Characterization of the ATP-inhibited K+ current in canine coronary smooth muscle cells. Pflugers Arch. 1994. 427:110–120.
Article15. Ashcroft FM. Adenosine-5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988. 11:97–118.16. Weiss J, Shine KI. Effects of heart rate on extracellular [K+] accumulation during myocardial ischemia. Am J Physiol. 1986. 250:H982–H991.
Article17. Hill JL, Gettes LS. Effect of acute coronary artery acclusion on local myocardial extracellular K+ activity in swine. Circulation. 1980. 61:768–778.
Article18. Hirche H, Franz C, Bös L, Bissig R, Lang R, Schramm M. Myocardial extracellular K+ and H+ increase and noradrenaline release as possible cause of early arrythmias following acute coronary artery occlusion In pigs. J Mol Cell Cardiol. 1980. 12:579–593.
Article19. Akil H, Owens C, Gutstein H, Taylor L, Curran E, Watson S. Endogenous opioids: overview and current issues. Drug Alcohol Depend. 1998. 51:127–140.
Article20. Ventura C, Spurgeon H, Lakatta EG, Guarnieri C, Capogrossi MC. kappa and delta opioid receptor stimulation affects cardiac myocyte function and Ca2+ release from an intracellular pool in myocytes and neurons. Circ Res. 1992. 70:66–81.
Article21. Wittert G, Hope P, Pyle D. Tissue distribution of opioid receptor gene expression in the rat. Biochem Biophys Res Commun. 1996. 218:877–881.
Article22. Holaday JW. Cardiovascular effects of endogenous opiate systems. Annu Rev Pharmacol Toxicol. 1983. 23:541–594.
Article23. Weil J, Eschenhagen T, Fleige G, Mittmann C, Orthey E, Schloz H. Localization of preproenkephalin mRNA in rat heart: selective gene expression in left ventricular myocardium. Am J Physiol. 1998. 275:H378–H384.24. Boluyt MO, Younes A, Caffrey JL, O'Neill L, Barron BA, Crow MT, et al. Age-associated increase in rat cardiac opioid production. Am J Physiol. 1993. 265:H212–H218.
Article25. Mantelli L, Corti V, Ledda F. On the presence of opioid receptors in guinea-pig ventricular tissue. Gen Pharmacol. 1987. 18:309–313.
Article26. Schultz JE, Rose E, Yao Z, Gross GJ. Evidence for involvement of opioid receptors in ischemic preconditioning in rat hearts. Am J Physiol. 1995. 268:H2157–H2161.
Article27. Takasaki Y, Wolff RA, Chien GL, Van Winkle DM. Met5-enkephalin protects isolated adult rabbit cardiomyocytes via delta-opioid receptors. Am J Phyiol. 1999. 277:H2442–H2450.28. Wild KD, Vanderah T, Mosberg HI, Porreca F. Opioid delta receptor subtypes are associated with different potassium channels. Eur J Pharmacol. 1991. 193:135–136.
Article29. Patil BM, Thakker PR. Glibenclamide antagonizes the inhibitory effect of morphine on gall bladder emptying. J Pharm Pharmacol. 1996. 48:320–322.
Article30. Amstead WM. Nociceptin/orphanin FQ dilates pial arteris by K(ATP) and K(ca) channel activation. Brain Res. 1999. 835:315–323.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of PGI2 on the ATP-sensitive Potassium Channel Activity in the Isolated Rat Cardiac Myocytes
- Effects of Endothelium-derived Relaxing Factors on the Regulation of ATP-sensitive Potassium Channel Activity in Cardiac Myocytes
- Activation of the Cardiac ATP-Sensitive K+Channel by KR-30816,Newly Synthesized Potassium Channel Opener
- The effects of intracellular monocarboxylates on the ATP-sensitive potassium channels in rabbit ventricular myocytes
- The Effect of Trifluoroacetic Acid, a Metabolite of Isoflurane on the ATP-sensitive Potassium Channel in Rabbit Ventricular Myocytes