J Bone Metab.
2015 Nov;22(4):167-174. 10.11005/jbm.2015.22.4.167.
Position Statement: Drug Holiday in Osteoporosis Treatment with Bisphosphonates in South Korea
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 2Department of Orthopedic Surgery, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Department of Obstetrics and Gynecology, Soonchunhyang University College of Medicine, Bucheon, Korea.
- 4Department of Orthopedic Surgery, Guro Hospital, Korea University College of Medicine, Seoul, Korea.
- 5Department of Obstetrics and Gynecology, Guro Hospital, Korea University College of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea.
- 7Division of Endocrinology and Metabolism, Department of Internal Medicine, Soonchunhyang University College of Medicine, Seoul, Korea. byundw@schmc.ac.kr
- KMID: 2170124
- DOI: http://doi.org/10.11005/jbm.2015.22.4.167
Abstract
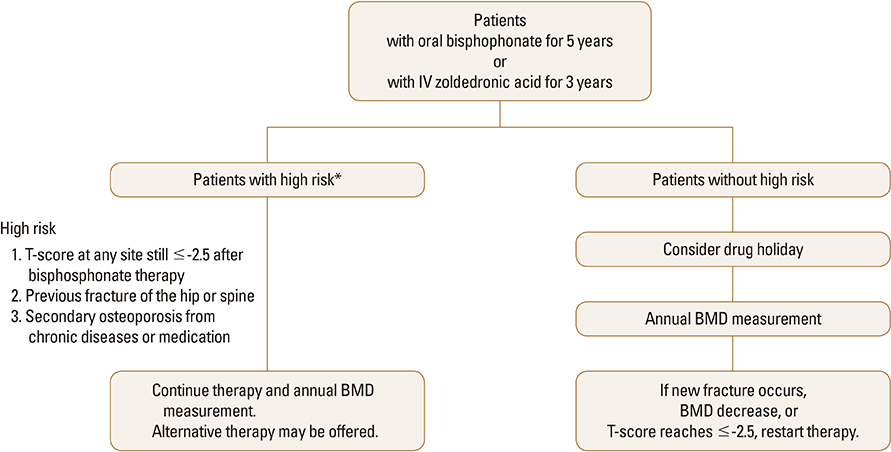
- Bisphosphonates have been widely used in the treatment of osteoporosis with robust data from many placebo-controlled trials demonstrating its efficacy in fracture risk reduction over 3 to 5 years of treatment. Although bisphosphonates are generally safe and well tolerated, concerns have emerged about the adverse effects related to its long-term use, including osteonecrosis of the jaw and atypical femur fractures. Because bisphosphonates are incorporated into the skeleton and continue to exert an anti-resorptive effect for a period of time after the discontinuation of drugs, the concept of a "drug holiday" has emerged, whereby the risk of adverse effects might be decreased while the patient still benefits from anti-fracture efficacy. As randomized clinical trial evidence is not yet available on who may qualify for a drug holiday, there is considerable controversy regarding the selection of candidates for the drug holiday and monitoring during a drug holiday, both of which should be based on individual assessments of risk and benefit. This statement will provide suggestions for clinicians in South Korea on the identification of possible candidates and monitoring during a bisphosphonate drug holiday.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Pharmacologic treatment of osteoporosis
Yong-Ki Min
J Korean Med Assoc. 2016;59(11):847-856. doi: 10.5124/jkma.2016.59.11.847.Position Statement on the Use of Bone Turnover Markers for Osteoporosis Treatment
So Young Park, Seong Hee Ahn, Jun-Il Yoo, Youn-Jee Chung, Yun Kyung Jeon, Byung-Ho Yoon, Ha Young Kim, Seung Hun Lee, Jehoon Lee, Seongbin Hong
J Bone Metab. 2019;26(4):213-224. doi: 10.11005/jbm.2019.26.4.213.Incidence Rate of Atypical Femoral Fracture after Bisphosphonates Treatment in Korea
Young-Kyun Lee, Soyeon Ahn, Kyoung Min Kim, Chang Suk Suh, Kyung-Hoi Koo
J Korean Med Sci. 2018;33(5):. doi: 10.3346/jkms.2018.33.e38.
Reference
-
1. Russell RG, Watts NB, Ebetino FH, et al. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008; 19:733–759.
Article2. Eastell R, Walsh JS, Watts NB, et al. Bisphosphonates for postmenopausal osteoporosis. Bone. 2011; 49:82–88.
Article3. Korean Endocrine Society. Osteoporosis fact sheet 2014. 2014. cited by 2015 September 1. Available from: http://www.endocrinology.or.kr/image/main/kor_Osteoporosis_Fact_Sheet2014.pdf.4. Khan AA, Morrison A, Hanley DA, et al. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res. 2015; 30:3–23.5. Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014; 29:1–23.
Article6. Bonnick SL. Going on a drug holiday? J Clin Densitom. 2011; 14:377–383.
Article7. Choi HJ, Im JA, Kim SH. Changes in bone markers after once-weekly low-dose alendronate in postmenopausal women with moderate bone loss. Maturitas. 2008; 60:170–176.
Article8. Li M, Zhang ZL, Liao EY, et al. Effect of low-dose alendronate treatment on bone mineral density and bone turnover markers in Chinese postmenopausal women with osteopenia and osteoporosis. Menopause. 2013; 20:72–78.
Article9. Uchida S, Taniguchi T, Shimizu T, et al. Therapeutic effects of alendronate 35 mg once weekly and 5 mg once daily in Japanese patients with osteoporosis: a double-blind, randomized study. J Bone Miner Metab. 2005; 23:382–388.
Article10. Ro C, Cooper O. Bisphosphonate drug holiday: choosing appropriate candidates. Curr Osteoporos Rep. 2013; 11:45–51.
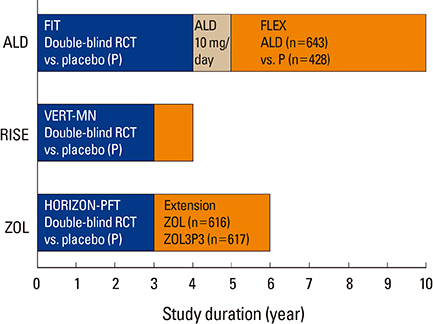
Article11. Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2012; 27:243–254.
Article12. Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006; 296:2927–2938.
Article13. Watts NB, Chines A, Olszynski WP, et al. Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int. 2008; 19:365–372.
Article14. Schilcher J, Koeppen V, Aspenberg P, et al. Risk of atypical femoral fracture during and after bisphosphonate use. N Engl J Med. 2014; 371:974–976.
Article15. Schilcher J, Michaëlsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med. 2011; 364:1728–1737.
Article16. Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010; 95:1555–1565.
Article17. Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010; 16:Suppl 3. 1–37.
Article18. Whitaker M, Guo J, Kehoe T, et al. Bisphosphonates for osteoporosis--where do we go from here? N Engl J Med. 2012; 366:2048–2051.
Article19. McClung M, Harris ST, Miller PD, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med. 2013; 126:13–20.
Article20. Black DM, Bauer DC, Schwartz AV, et al. Continuing bisphosphonate treatment for osteoporosis--for whom and for how long? N Engl J Med. 2012; 366:2051–2053.
Article21. Nancollas GH, Tang R, Phipps RJ, et al. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone. 2006; 38:617–627.
Article22. Rodan G, Reszka A, Golub E, et al. Bone safety of long-term bisphosphonate treatment. Curr Med Res Opin. 2004; 20:1291–1300.
Article23. Strom O, Landfeldt E, Garellick G. Residual effect after oral bisphosphonate treatment and healthy adherer effects--the Swedish Adherence Register Analysis (SARA). Osteoporos Int. 2015; 26:315–325.
Article24. Bonnick SL, Shulman L. Monitoring osteoporosis therapy: bone mineral density, bone turnover markers, or both? Am J Med. 2006; 119:S25–S31.
Article25. Bauer DC, Schwartz A, Palermo L, et al. Fracture prediction after discontinuation of 4 to 5 years of alendronate therapy: the FLEX study. JAMA Intern Med. 2014; 174:1126–1134.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Duration of Bisphosphonate Treatment
- The Trend in the Sales of Menopausal Hormone and Other Osteoporosis Medications in South Korea from 2016 to 2019
- The effect of drug holiday before tooth extraction on the development of medication-related osteonecrosis of the jaw in cancer patients receiving intravenous bisphosphonates
- Palliative Care and Hospice for Heart Failure Patients: Position Statement From the Korean Society of Heart Failure
- Physicians’ Attitudes on Management of Osteopenia in South Korea