J Bacteriol Virol.
2015 Dec;45(4):354-363. 10.4167/jbv.2015.45.4.354.
Eosinophils are Required for Immune Responses Induced by Oral Immunization
- Affiliations
-
- 1Department of Microbiology, School of Medicine, Gachon University, Incheon Korea. yjjung@gachon.ac.kr
- KMID: 2168719
- DOI: http://doi.org/10.4167/jbv.2015.45.4.354
Abstract
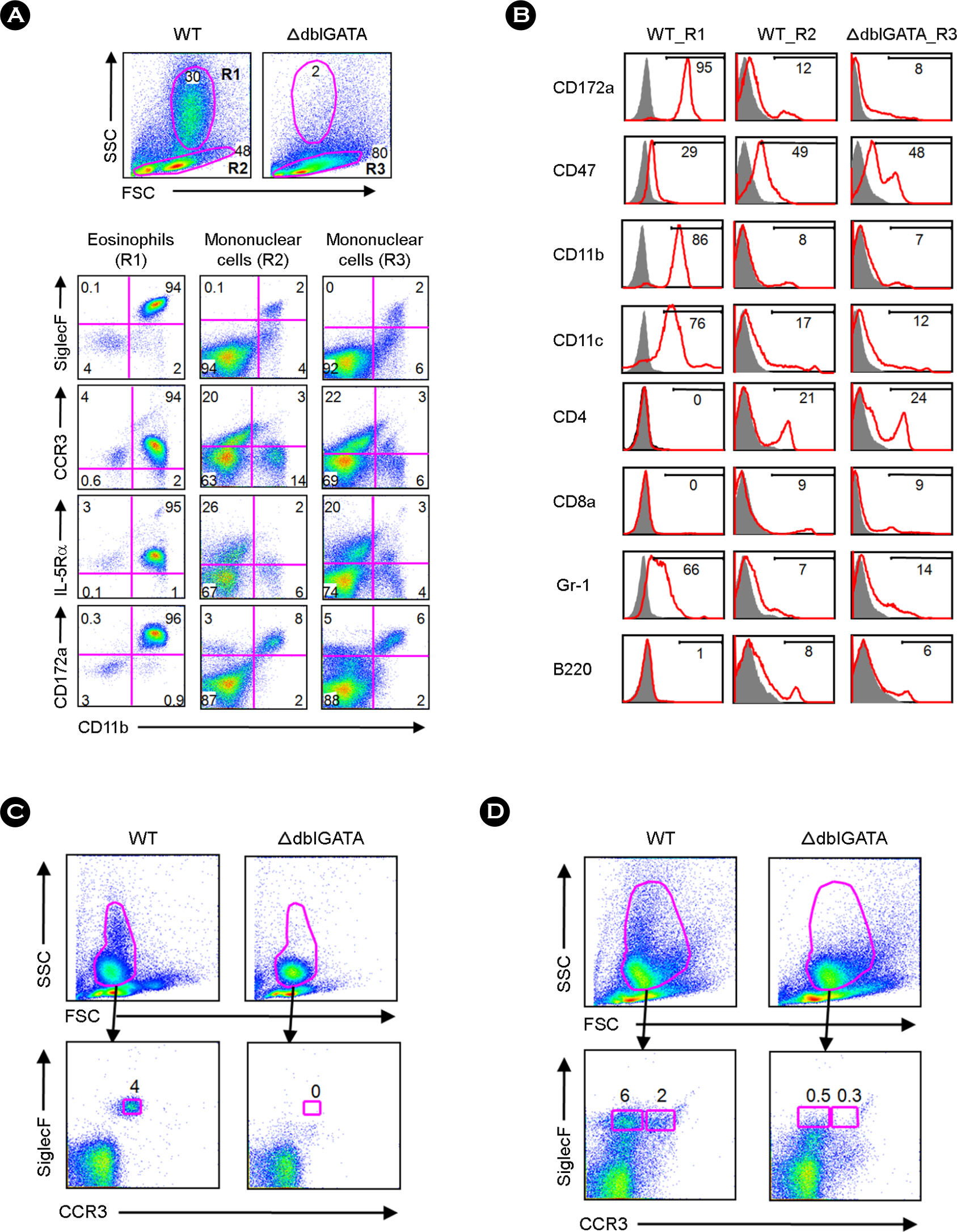
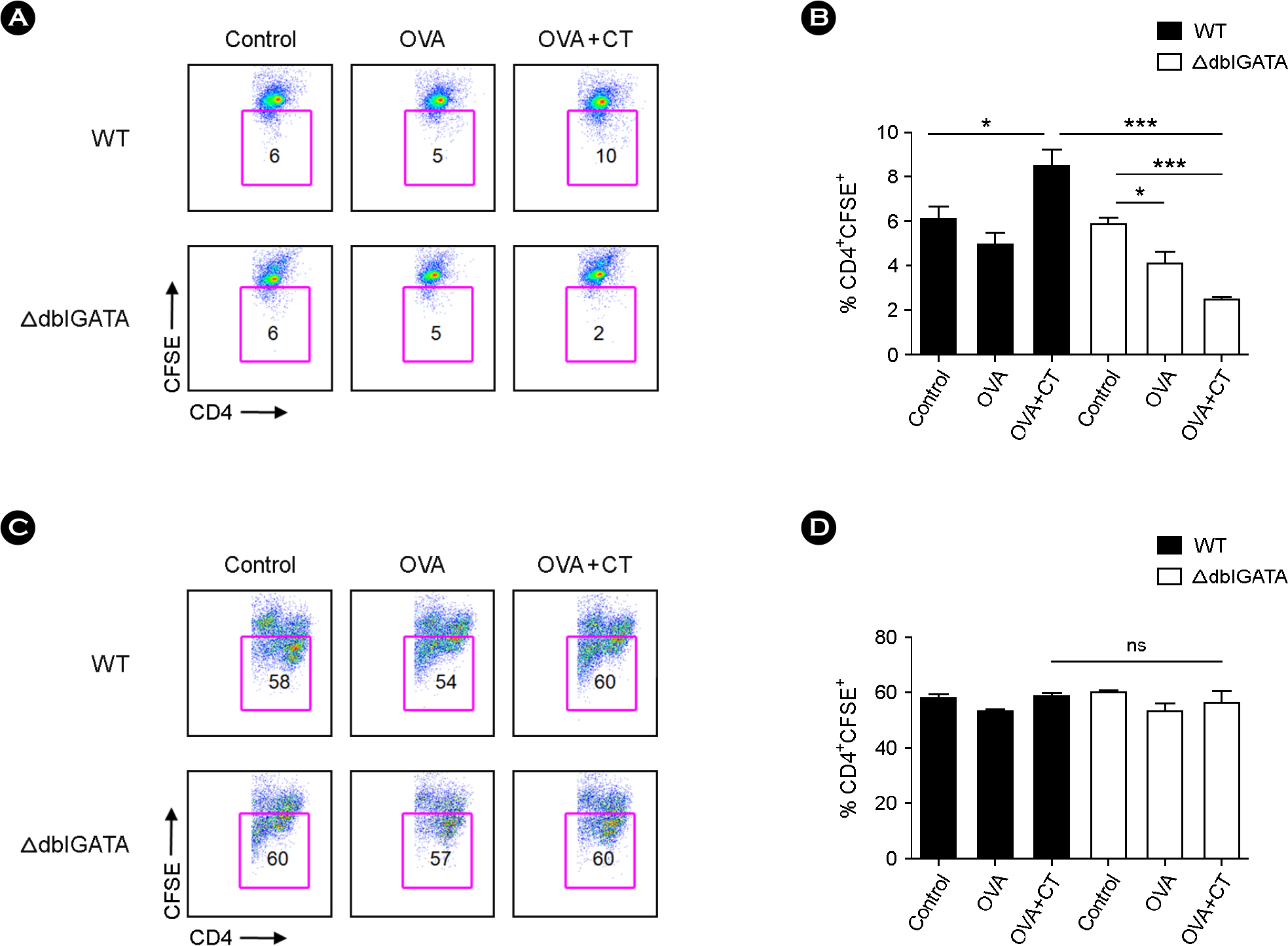
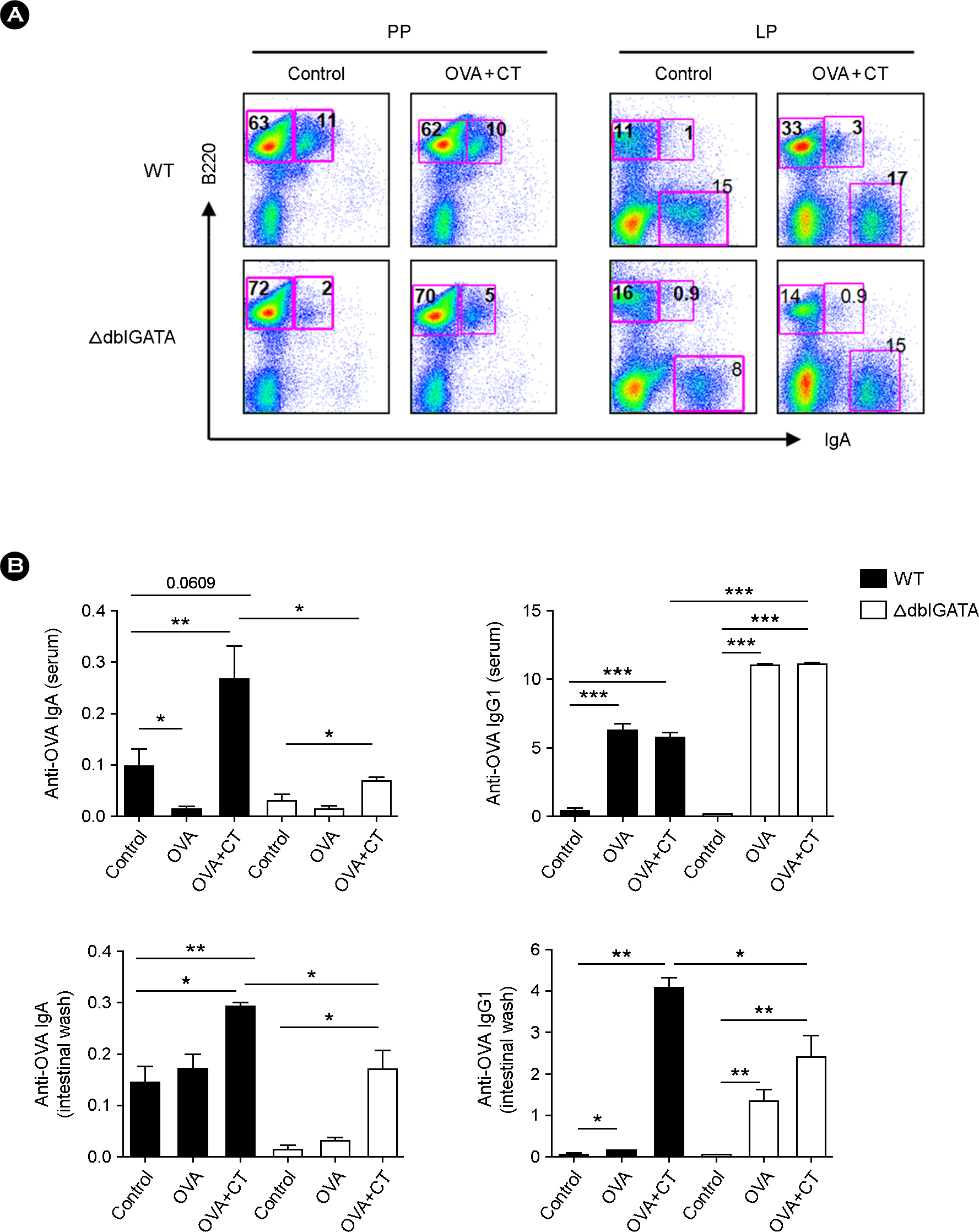
- Eosinophils are multifunctional leukocytes that reside in several tissues, most abundantly in the small intestinal lamina propria under the steady state. To date, the phenotypic and functional characteristics of small intestinal eosinophils have remained poorly understood. In this study, we found that proliferation of ovalbumin (OVA)-specific CD4+ T cells isolated from the mesenteric lymph nodes of eosinophil-deficient DeltadblGATA mice were decreased relative to wild-type mice after oral immunization with OVA and cholera toxin (CT), the typical mucosal adjuvant that induces CD4+ T cell-dependent responses. DeltadblGATA mice showed reduced mucosal secretion of OVA-specific IgA and IgG1 while maintaining a systemic level of anti-OVA IgG1 upon oral immunization with OVA and CT. These findings suggest that eosinophils might have a role in the modulation of T cell-mediated immune responses including mucosal antibody responses in the gastrointestinal tract following oral immunization.
MeSH Terms
Figure
Cited by 2 articles
-
Eosinophils Regulate Type 2 Immune Responses Following Infection with the Nematode
Trichinella spiralis
Jayoung Koo Koo, YunJae Jung
J Bacteriol Virol. 2016;46(4):295-302. doi: 10.4167/jbv.2016.46.4.295.Eosinophils and Type 2 Cytokine Signaling in Macrophages Support the Biogenesis of Cold-induced Beige Fat
YunJae Jung
J Bacteriol Virol. 2016;46(1):44-46. doi: 10.4167/jbv.2016.46.1.44.
Reference
-
1). Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006; 24:147–74.
Article2). Jung Y, Rothenberg ME. Roles and regulation of gastrointestinal eosinophils in immunity and disease. J Immunol. 2014; 193:999–1005.
Article3). Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol. 2004; 113:11–28.
Article4). Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, et al. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008; 205:79–90.
Article5). Hirasawa R, Shimizu R, Takahashi S, Osawa M, Takayanagi S, Kato Y, et al. Essential and instructive roles of GATA factors in eosinophil development. J Exp Med. 2002; 195:1379–86.
Article6). Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, et al. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002; 195:1387–95.7). Mishra A, Hogan SP, Lee JJ, Foster PS, Rothenberg ME. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest. 1999; 103:1719–27.
Article8). Carlens J, Wahl B, Ballmaier M, Bulfone-Paus S, Förster R, Pabst O. Common gamma-chain-dependent signals confer selective survival of eosinophils in the murine small intestine. J Immunol. 2009; 183:5600–7.9). Verjan Garcia N, Umemoto E, Saito Y, Yamasaki M, Hata E, Matozaki T, et al. SIRPalpha/CD172a Regulates Eosinophil Homeostasis. J Immunol. 2011; 187:2268–77.10). Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003; 3:331–41.
Article11). Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu Rev Immunol. 2010; 28:243–73.
Article12). Elson CO, Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984; 132:2736–41.13). Lycke N, Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986; 59:301–8.14). Bergqvist P, Gärdby E, Stensson A, Bemark M, Lycke NY. Gut IgA class switch recombination in the absence of CD40 does not occur in the lamina propria and is independent of germinal centers. J Immunol. 2006; 177:7772–83.
Article15). Jung Y, Wen T, Mingler MK, Caldwell JM, Wang YH, Chaplin DD, et al. IL-1beta in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol. 2015; 8:930–42.16). Westlund J, Livingston M, Fahlén-Yrlid L, Oldenborg PA, Yrlid U. CD47-deficient mice have decreased production of intestinal IgA following oral immunization but a maintained capacity to induce oral tolerance. Immunology. 2012; 135:236–44.
Article17). Yamamoto M, Rennert P, McGhee JR, Kweon MN, Yamamoto S, Dohi T, et al. Alternate mucosal immune system: organized Peyer's patches are not required for IgA responses in the gastrointestinal tract. J Immunol. 2000; 164:5184–91.
Article18). Orr N, Robin G, Cohen D, Arnon R, Lowell GH. Immunogenicity and efficacy of oral or intranasal Shigella flexneri 2a and Shigella sonnei proteosome-lipopolysaccharide vaccines in animal models. Infect Immun. 1993; 61:2390–5.
Article19). Seiffert M, Cant C, Chen Z, Rappold I, Brugger W, Kanz L, et al. Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood. 1999; 94:3633–43.
Article20). Xu-Amano J, Kiyono H, Jackson RJ, Staats HF, Fujihashi K, Burrows PD, et al. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J Exp Med. 1993; 178:1309–20.
Article21). Cho KA, Suh JW, Sohn JH, Park JW, Lee H, Kang JL, et al. IL-33 induces Th17-mediated airway inflammation via mast cells in ovalbumin-challenged mice. Am J Physiol Lung Cell Mol Physiol. 2012; 302:L429–40.
Article22). Chu VT, Beller A, Rausch S, Strandmark J, Zänker M, Arbach O, et al. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity. 2014; 40:582–93.
Article23). Wen T, Mingler MK, Blanchard C, Wahl B, Pabst O, Rothenberg ME. The pan-B cell marker CD22 is expressed on gastrointestinal eosinophils and negatively regulates tissue eosinophilia. J Immunol. 2012; 188:1075–82.
Article24). Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008; 1:11–22.
Article25). Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008; 28:740–50.
Article26). Cazac BB, Roes J. TGF-beta receptor controls B cell responsiveness and induction of IgA in vivo. Immunity. 2000; 13:443–51.27). Mishra A, Hogan SP, Brandt EB, Rothenberg ME. Peyer's patch eosinophils: identification, characterization, and regulation by mucosal allergen exposure, interleukin-5, and eotaxin. Blood. 2000; 96:1538–44.
Article28). Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008; 9:769–76.
Article29). Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006; 314:1157–60.
Article30). Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001; 413:639–43.
Article31). Yuvaraj S, Dijkstra G, Burgerhof JG, Dammers PM, Stoel M, Visser A, et al. Evidence for local expansion of IgA plasma cell precursors in human ileum. J Immunol. 2009; 183:4871–8.
Article32). He B, Raab-Traub N, Casali P, Cerutti A. EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching. J Immunol. 2003; 171:5215–24.
Article33). Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002; 3:822–9.
Article34). Xu W, He B, Chiu A, Chadburn A, Shan M, Buldys M, et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat Immunol. 2007; 8:294–303.
Article35). Chu VT, Fröhlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. 2011; 12:151–9.
Article36). Schaffeler MP, Brokenshire JS, Snider DP. Detection of precursor Th cells in mesenteric lymph nodes after oral immunization with protein antigen and cholera toxin. Int Immunol. 1997; 9:1555–62.
Article37). Demeure CE, Yang LP, Byun DG, Ishihara H, Vezzio N, Delespesse G. Human naive CD4 T cells produce interleukin-4 at priming and acquire a Th2 phenotype upon repetitive stimulations in neutral conditions. Eur J Immunol. 1995; 25:2722–5.
Article38). Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci U S A. 1993; 90:10188–92.39). Thompson CB, Lindsten T, Ledbetter JA, Kunkel SL, Young HA, Emerson SG, et al. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci U S A. 1989; 86:1333–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of systemic and mucosal immune responses in mice administered with recombinant Salmonella Typhimurium expressing IutA protein
- Enhancement of DNA Vaccine-induced Immune Responses by Influenza Virus NP Gene
- Investigating immunization via the esophagus: carrageenan’s impact on immune activation
- Effect of Capsaicin on Immune Responses, Anaphylaxis and Tumorigenesis in Mice
- Regulatory Eosinophils in Inflammation and Metabolic Disorders