J Bacteriol Virol.
2012 Dec;42(4):346-352. 10.4167/jbv.2012.42.4.346.
Inhibition of Transfer Infection of Epstein-Barr Virus to Epithelial Cells by Integrin beta6 siRNA
- Affiliations
-
- 1Research Institute of Immunobiology, Department of Biomedical Sciences, College of Medicine, The Catholic University of Seoul, Korea. sukklee@catholic.ac.kr
- KMID: 2168670
- DOI: http://doi.org/10.4167/jbv.2012.42.4.346
Abstract
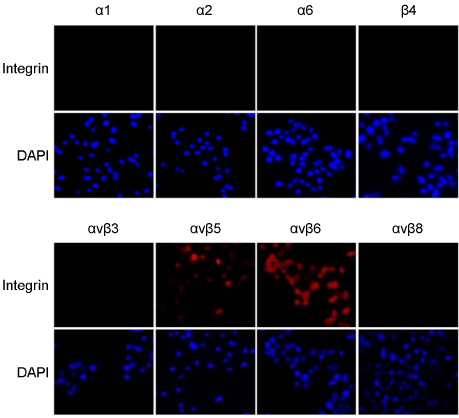
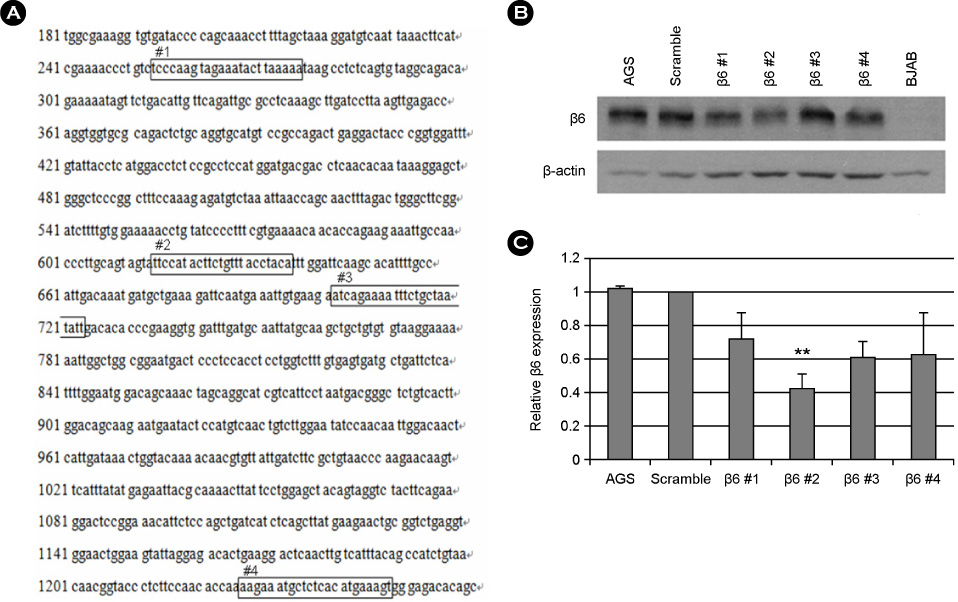
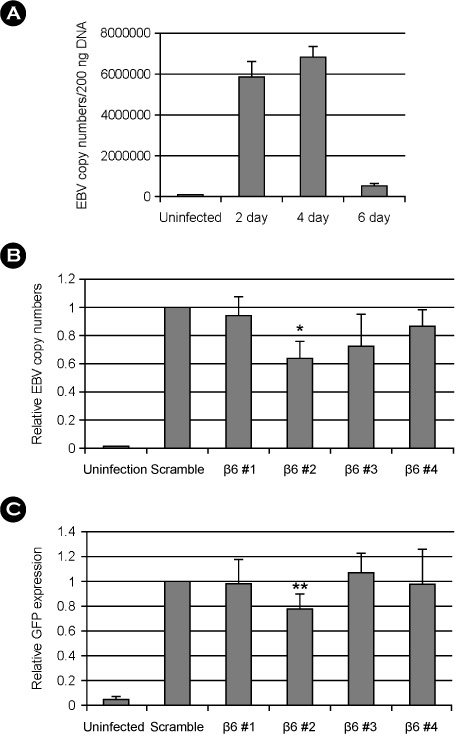
- Epstein-Barr virus (EBV) establishes a latent infection in greater than 90% of the world's adult population and associates with various tumors. EBV primarily infects epithelial cells and B cell in vivo. Mechanism of EBV infection in B cells is known to involve binding of EBV glycoprotein gp350 to CD21 on B cell surface. Epithelial cells are infected with EBV even though most of epithelial cells do not express CD21. Recently, integrin alphavbeta5, alphavbeta6 and alphavbeta8 on epithelial cells were reported to facilitate EBV infection by interacting with gHgL complex. We examined the expression profile of integrins known to be expressed on epithelial cells. Integrin alphavbeta5 and alphavbeta6, but not alphavbeta8 were detected in a gastric epithelial cell line, AGS. We then tested whether siRNAs specific to beta6 can inhibit EBV infection of epithelial cells. One among the four tested siRNAs significantly reduced beta6 expression and suppressed transfer infection of EBV to AGS cells. Our data suggest that siRNAs to integrins might be useful to control EBV infection to epithelial cells.
MeSH Terms
Figure
Reference
-
1. Klein E. The complexity of the Epstein-Barr virus infection in humans. Pathol Oncol Res. 1998. 4:3–7.
Article2. Rickinson AB, Kieff E. Fields BN, Knipe DM, Howley PM, editors. Epstein-Barr virus. Fields Virology. 1996. 3rd ed. Philadelphia: Lippincott-Raven;2397–2446.3. Shibata D, Weiss LM. Epstein-Barr virus-associated gastric adenocarcinoma. Am J Pathol. 1992. 140:769–774.4. Takada K. Epstein-Barr virus and gastric carcinoma. Mol Pathol. 2000. 53:255–261.
Article5. Niedobitek G, Agathanggelou A, Herbst H, Whitehead L, Wright DH, Young LS. Epstein-Barr virus (EBV) infection in infectious mononucleosis: virus latency, replication and phenotype of EBV-infected cells. J Pathol. 1997. 182:151–159.
Article6. Hutt-Fletcher LM. Epstein-Barr virus entry. J Virol. 2007. 81:7825–7832.
Article7. Li Q, Spriggs MK, Kovats S, Turk SM, Comeau MR, Nepom B, et al. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J Virol. 1997. 71:4657–4662.
Article8. Borza CM, Morgan AJ, Turk SM, Hutt-Fletcher LM. Use of gHgL for attachment of Epstein-Barr virus to epithelial cells compromises infection. J Virol. 2004. 78:5007–5014.
Article9. Molesworth SJ, Lake CM, Borza CM, Turk SM, Hutt-Fletcher LM. Epstein-Barr virus gH is essential for penetration of B cells but also plays a role in attachment of virus to epithelial cells. J Virol. 2000. 74:6324–6332.
Article10. Chesnokova LS, Nishimura SL, Hutt-Fletcher LM. Fusion of epithelial cells by Epstein-Barr virus proteins is triggered by binding of viral glycoproteins gHgL to integrins alphavbeta6 or alphavbeta8. Proc Natl Acad Sci U S A. 2009. 106:20464–20469.
Article11. Chesnokova LS, Hutt-Fletcher LM. Fusion of Epstein-Barr virus with epithelial cells can be triggered by αvβ5 in addition to αvβ6 and αvβ8, and integrin binding triggers a conformational change in glycoproteins gHgL. J Virol. 2011. 85:13214–13223.
Article12. Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999. 285:1028–1032.
Article13. Gilcrease MZ. Integrin signaling in epithelial cells. Cancer Lett. 2007. 247:1–25.
Article14. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002. 110:673–687.15. Seo NS, Zeng CQ, Hyser JM, Utama B, Crawford SE, Kim KJ, et al. Integrins alpha1beta1 and alpha2beta1 are receptors for the rotavirus enterotoxin. Proc Natl Acad Sci U S A. 2008. 105:8811–8818.16. Fang IM, Yang CH, Yang CM, Chen MS. Overexpression of integrin alpha6 and beta4 enhances adhesion and proliferation of human retinal pigment epithelial cells on layers of porcine Bruch's membrane. Exp Eye Res. 2009. 88:12–21.
Article17. Yoshiyama H, Imai S, Shimizu N, Takada K. Epstein-Barr virus infection of human gastric carcinoma cells: implication of the existence of a new virus receptor different from CD21. J Virol. 1997. 71:5688–5691.
Article18. Speck P, Kline KA, Cheresh P, Longnecker R. Epstein-Barr virus lacking latent membrane protein 2 immortalizes B cells with efficiency indistinguishable from that of wild-type virus. J Gen Virol. 1999. 80:2193–2203.
Article19. Seo JS, Cho NY, Kim HR, Tsurumi T, Jang YS, Lee WK, et al. Cell cycle arrest and lytic induction of EBV-transformed B lymphoblastoid cells by a histone deacetylase inhibitor, Trichostatin A. Oncol Rep. 2008. 19:93–98.
Article20. Fingeroth JD, Weis JJ, Tedder TF, Strominger JL, Biro PA, Fearon DT. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci U S A. 1984. 81:4510–4514.
Article21. Shannon-Lowe CD, Neuhierl B, Baldwin G, Rickinson AB, Delecluse HJ. Resting B cells as a transfer vehicle for Epstein-Barr virus infection of epithelial cells. Proc Natl Acad Sci U S A. 2006. 103:7065–7070.
Article22. Shimizu N, Yoshiyama H, Takada K. Clonal propagation of Epstein-Barr virus (EBV) recombinants in EBV-negative Akata cells. J Virol. 1996. 70:7260–7263.
Article23. Kieff E. Fields BN, Knipe DM, Howley PM, editors. Epstein-Barr virus and its replication. Fundamental Virology. 1996. New York: Raven Press;1109–1162.24. Shapiro IM, Volsky DJ. Infection of normal human epithelial cells by Epstein-Barr virus. Science. 1983. 219:1225–1228.
Article25. Zhao R, Liu XQ, Wu XP, Liu YF, Zhang ZY, Yang GY, et al. Vascular endothelial growth factor (VEGF) enhances gastric carcinoma invasiveness via integrin alpha(v)beta6. Cancer Lett. 2010. 287:150–156.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Epstein-Barr Virus-Associated Hemophagocytic Syndrome with Ascites
- A Case of Epstein-Barr Virus Infection Presented as Evans Syndrome
- A Case of Epstein-Barr Virus-Associated Hemophagocytic Syndrome Demonstrated by In Situ Hybridization
- A Case of Epstein-Barr Virus-Positive Diffuse Large B-Cell Lymphoma Occurring in Thyroid Gland
- Guillain-Barré syndrome supervening on meningitis in primary Epstein-Barr virus infection