J Bacteriol Virol.
2011 Jun;41(2):65-76. 10.4167/jbv.2011.41.2.65.
Infectobesity: a New Area for Microbiological and Virological Research
- Affiliations
-
- 1Department of Biotechnology, The Catholic University, Gyeonggi-do, Korea. jhnam@catholic.ac.kr
- KMID: 2168623
- DOI: http://doi.org/10.4167/jbv.2011.41.2.65
Abstract
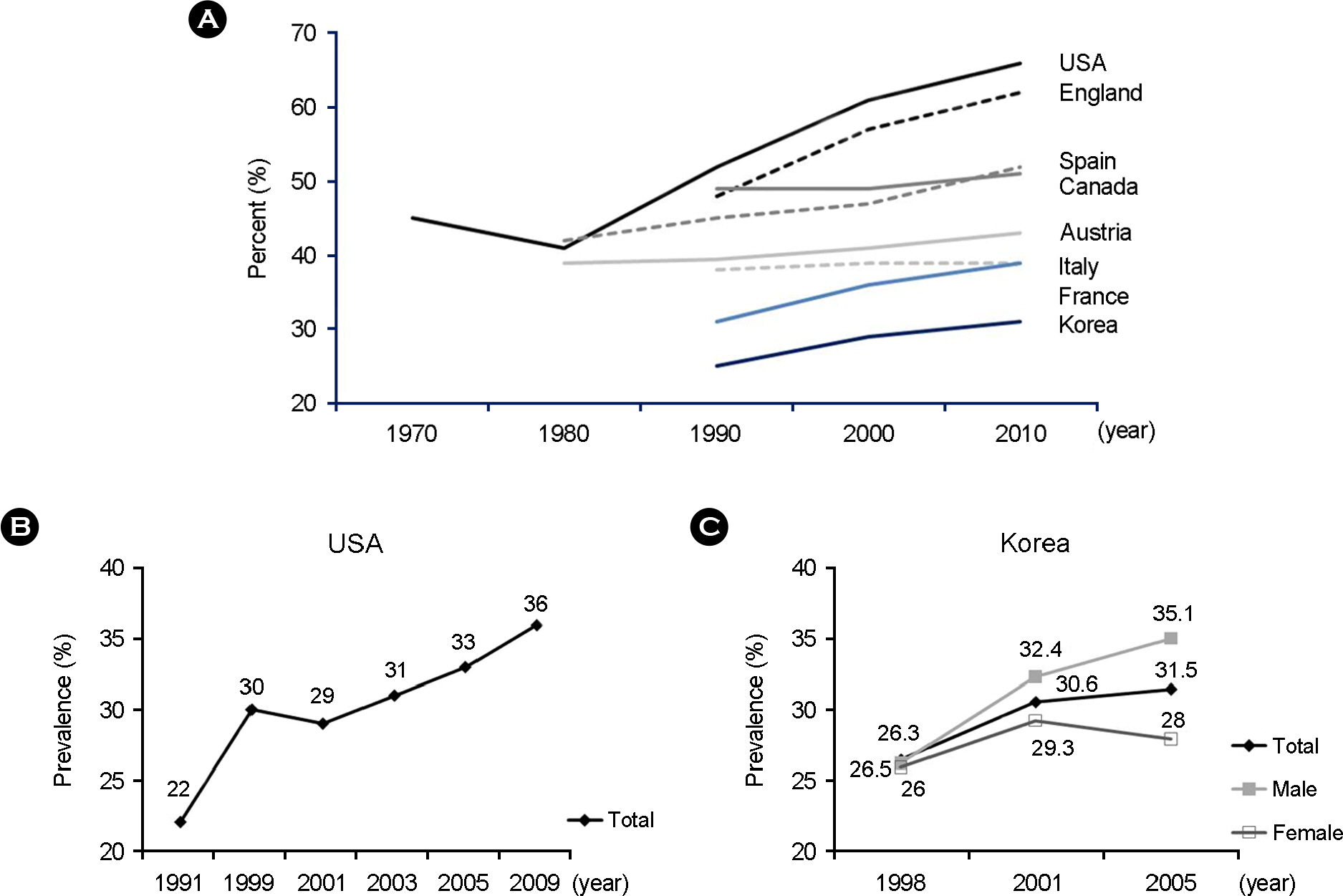
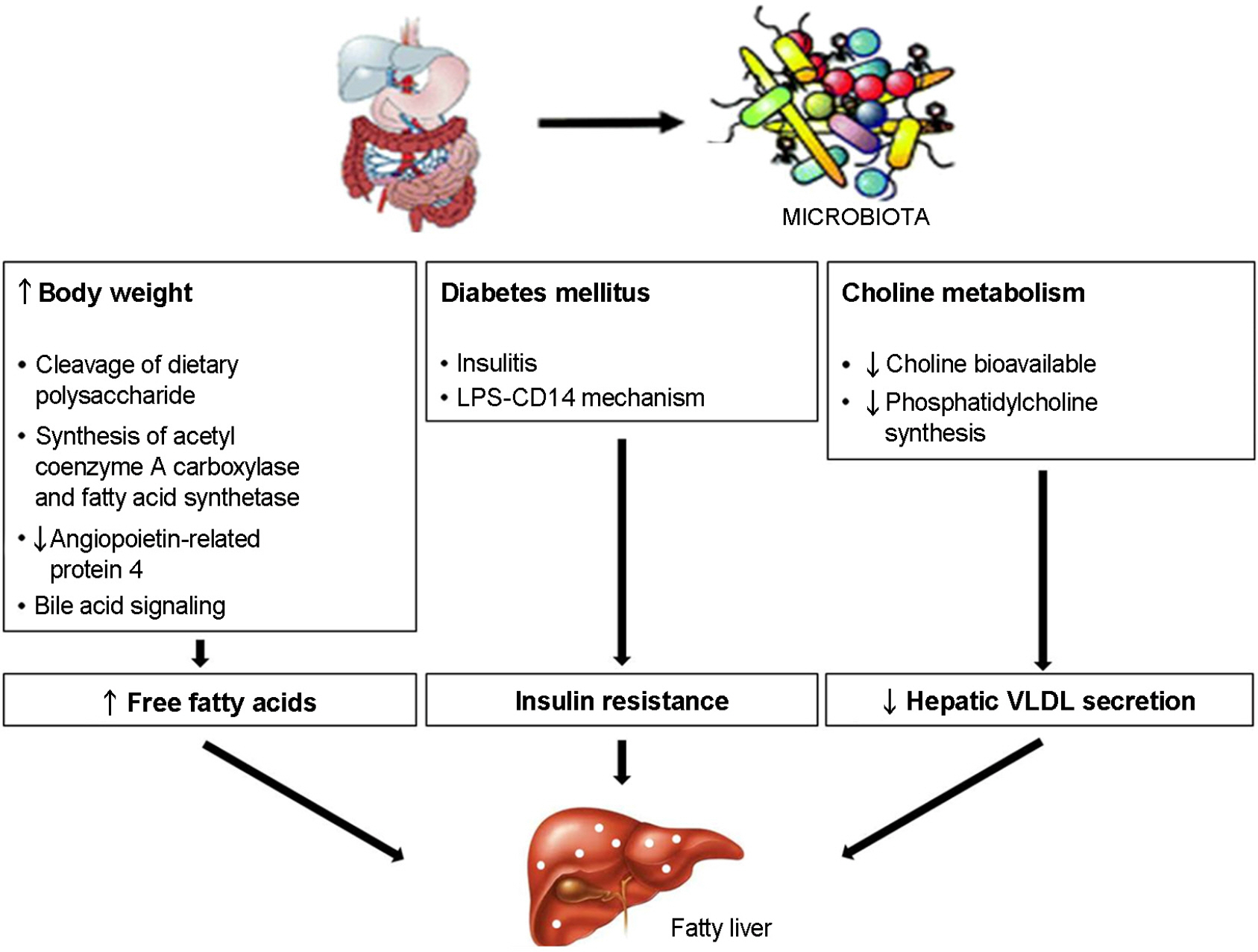
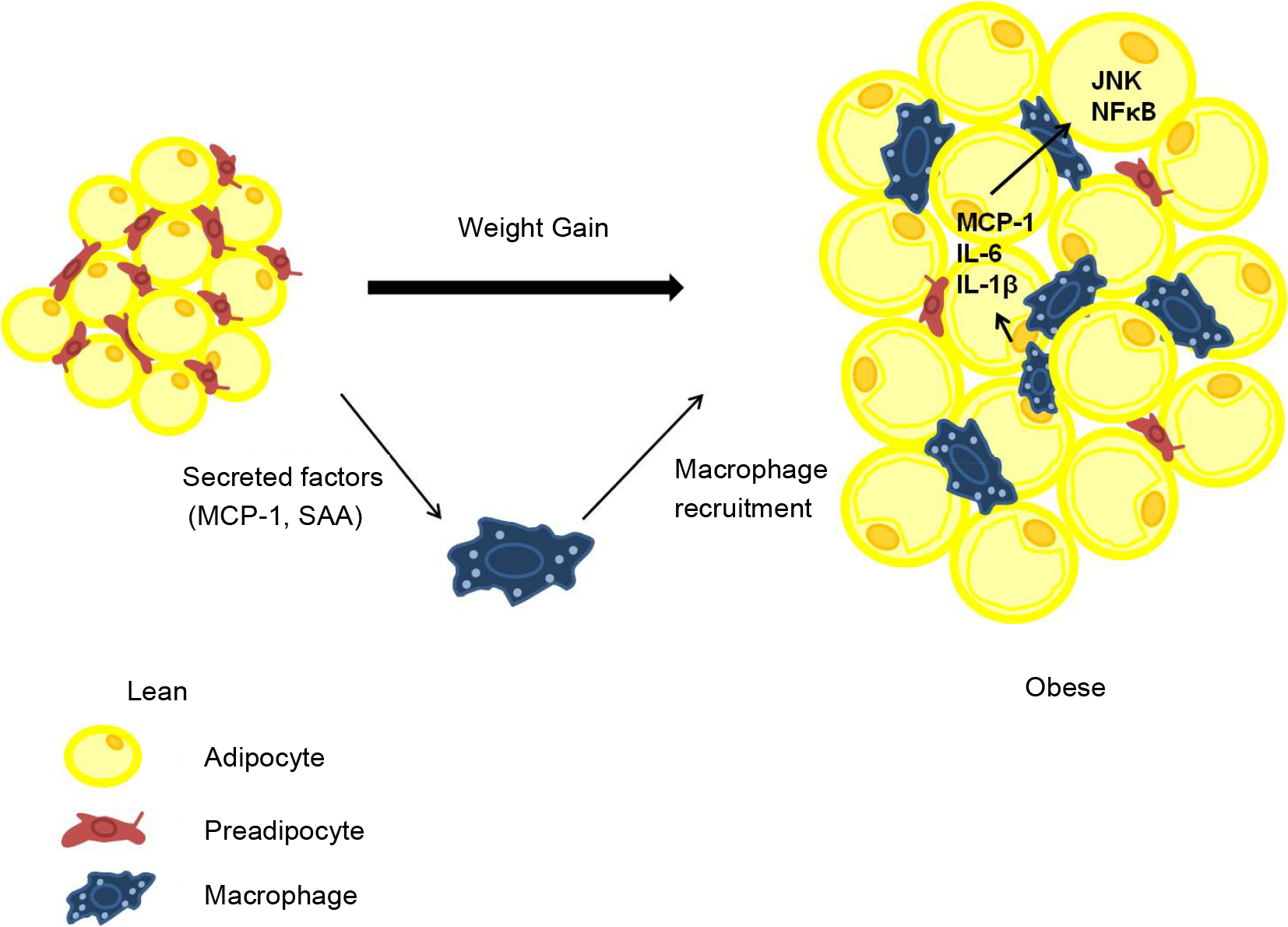
- Obesity is connected with numerous diseases, such as type 2 diabetes, atherosclerosis, cancer, and nervous system dysfunctions. Obesity is affected by genetic, environmental, and cultural factors. However, numerous studies indicate that several pathogens might cause obesity. This review discusses recent data and the characteristics of pathogens that are implicated in obesity. In particular, human adenovirus 36 (Ad36) is the most clearly implicated virus in human obesity. It was recently shown that obese groups from the USA, Korea, and Italy have a higher prevalence of serum antibodies against Ad36. The mechanisms of Ad36-induced obesity remain unclear. However, glucose uptake and inflammation are possible mechanisms of Ad36-induced obesity. Overall, this new understanding of causes of obesity has developed into the concept of 'infectobesity' and the possibility of developing a 'vaccine' or 'therapeutic agents' for obesity.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Roles of Enteric Microbial Composition and Metabolism in Health and Diseases
Jung Mogg Kim
Korean J Gastroenterol. 2013;62(4):191-205. doi: 10.4166/kjg.2013.62.4.191.Is Obesity One of Physiological Factors which Exert Influenza Virus-induced Pathology and Vaccine Efficacy?
Whajung Cho, Jae-Hwan Nam
J Bacteriol Virol. 2014;44(3):226-235. doi: 10.4167/jbv.2014.44.3.226.
Reference
-
1). Kim DM., Ahn CW., Nam SY. Prevalence of obesity in Korea. Obes Rev. 2005. 6:117–21.
Article2). Ogden CL., Carroll MD., Curtin LR., McDowell MA., Tabak CJ., Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006. 295:1549–55.
Article3). Jeong BG., Moon OR., Kim NS., Kang JH., Yoon TH., Lee SJ, et al. Socioeconimic costs of obesity for Korean adults. Korean J Prev Med. 2002. 35:1–12.4). Butte NF., Christiansen E., Sorensen TI. Energy imbalance underlying the development of childhood obesity. Obesity. 2007. 15:3056–66.5). Hebebrand J., Hinney A. Environmental and genetic risk factors in obesity. Child Adolesc Psychiatr Clin N Am. 2009. 18:83–94.
Article6). van Ginneken V., Sitnyakowsky L., Jeffery JE. Infectobesity: viral infections (especially with human adenovirus-36: Ad-36) may be a cause of obesity. Med Hypotheses. 2009. 72:383–8.
Article7). Pasarica M., Dhurandhar NV. Infectobesity: obesity of infectious origin. Adv Food Nutr Res. 2007. 52:61–102.
Article8). Dhurandhar NV. Infectobesity: obesity of infectious origin. J Nutr. 2001. 131:2794S–7S.
Article9). Dhurandhar NV. Atkinson RL. Obesity of infectious origin-A Review. GGH. 2004. 20:33–9.10). Greenway F. Virus-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2006. 290:R188–9.
Article11). Atkinson RL. Viruses as an etiology of obesity. Mayo Clin Proc. 2007. 82:1192–8.12). Detwiler LA. Scrapie. Rev Sci Tech. 1992. 11:491–537.
Article13). Kim YS., Carp RI., Callahan SM., Wisniewski HM. Scrapie-induced obesity in mice. J Infect Dis. 1987. 156:402–5.
Article14). Kim YS., Carp RI., Callahan SM., Wisniewski HM. Adrenal involvement in scrapie-induced obesity. Proc Soc Exp Biol Med. 1988. 189:21–7.
Article15). Carp RI., Kim YS., Callahan SM. Scrapie-induced alterations in glucose tolerance in mice. J Gen Virol. 1989. 70:827–35.
Article16). Kuo CC., Jackson LA., Campbell LA., Grayston JT. Chlamydia pneumoniae (TWAR). Clin Microbiol Rev. 1995. 8:451–61.17). Rantala A., Lajunen T., Juvonen R., Bloigu A., Paldanius M., Silvennoinen-Kassinen S, et al. Chlamydia pneumoniae infection is associated with elevated body mass index in young men. Epidemiol Infect. 2010. 138:1267–73.18). Koziolek M., Krzyzanowska-Swiniarska B., Maczyńska I., Miazgowski T., Giedrys-Kalemba S. The association between past Chlamydia pneumoniae infection and markers of chronic inflammation in obese women. Eur J Clin Microbiol Infect Dis. 2008. 27:415–21.19). Björkstén B., Sepp E., Julge K., Voor T., Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001. 108:516–20.
Article20). Guarner F., Malagelada JR. Gut flora in health and disease. Lancet. 2003. 361:512–9.
Article21). DiBaise JK., Zhang H., Crowell MD., Krajmalnik-Brown R., Decker GA., Rittmann BE. Gut microbiota and its possible relationship with obesity. Mayo Clin Proc. 2008. 83:460–9.
Article22). Sanz Y., Santacruz A., Gauffin P. Gut microbiota in obesity and metabolic disorders. Proc Nutr Soc. 2010. 69:434–41.
Article23). Turnbaugh PJ., Ley RE., Mahowald MA., Magrini V., Mardis ER., Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006. 444:1027–31.
Article24). Ley RE., Turnbaugh PJ., Klein S., Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006. 444:1022–3.25). Abu-Shanab A., Quigley EM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010. 7:691–701.
Article26). McCarthy AJ., Shaw MA., Goodman SJ. Pathogen evolution and disease emergence in carnivores. Proc Biol Sci. 2007. 274:3165–74.
Article27). Bernard A., Cohen R., Khuth ST., Vedrine B., Verlaeten O., Akaoka H, et al. Alteration of the leptin network in late morbid obesity induced in mice by brain infection with canine distemper virus. J Virol. 1999. 73:7317–27.
Article28). Münzel P., Koschel K. Alteration in phospholipid methylation and impairment of signal transmission in persistently paramyxovirus-infected C6 rat glioma cells. Proc Natl Acad Sci U S A. 1982. 79:3692–6.29). Nagashima K., Zabriskie JB., Lyons MJ. Virus-induced obesity in mice: association with a hypothalamic lesion. J Neuropathol Exp Neurol. 1992. 51:101–9.
Article30). Carter JK., Smith RE. Specificity of avian leukosis virus-induced hyperlipidemia. J Virol. 1984. 50:301–8.
Article31). Carter JK., Ow CL., Smith RE. Rous-associated virus type 7 induces a syndrome in chickens characterized by stunting and obesity. Infect Immun. 1983. 39:410–22.
Article32). Dhurandhar NV., Kulkarni PR., Ajinkya SM., Sherikar AA., Atkinson RL. Association of adenovirus infection with human obesity. Obes Res. 1997. 5:464–9.
Article33). Stitz L., Dietzschold B., Carbone KM. Immunopathogenesis of Borna disease. Curr Top Microbiol Immunol. 1995. 190:75–92.
Article34). Dauphin G., Legay V., Pitel PH., Zientara S. Borna disease: current knowledge and virus detection in France. Vet Res. 2002. 33:127–38.
Article35). Gosztonyi G., Ludwig H. Borna disease–neuropathology and pathogenesis. Curr Top Microbiol Immunol. 1995. 190:39–73.
Article36). Herden C., Herzog S., Richt JA., Nesseler A., Christ M., Failing K, et al. Distribution of Borna disease virus in the brain of rats infected with an obesity-inducing virus strain. Brain Pathol. 2000. 10:39–48.
Article37). Hirano N., Kao M., Ludwig H. Persistent, tolerant or subacute infection in Borna disease virus-infected rats. J Gen Virol. 1983. 64(Pt 7):1521–30.
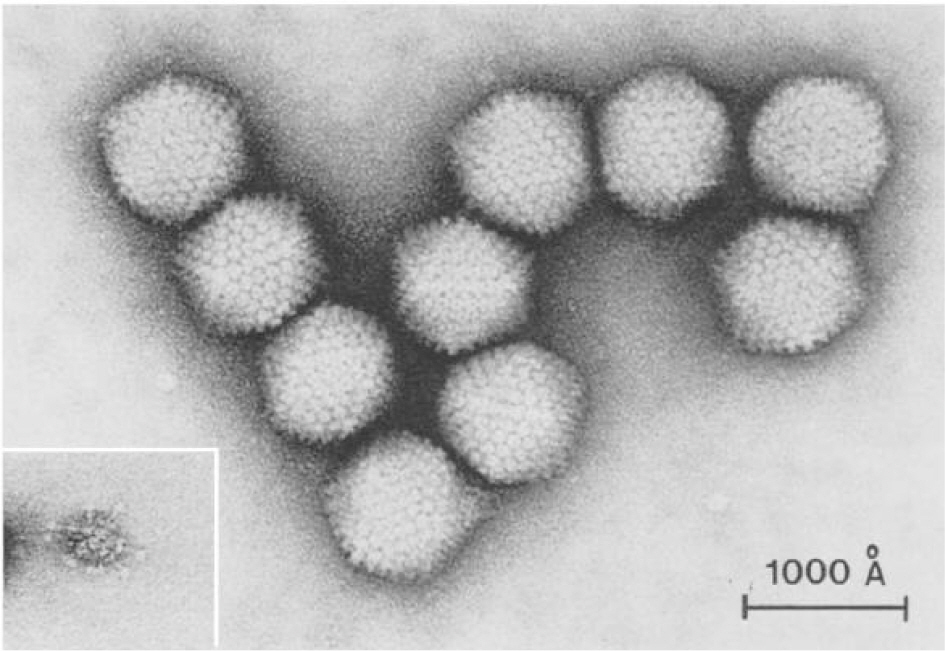
Article38). Wigand R., Gelderblom H., Wadell G. New human adenovirus (candidate adenovirus 36), a novel member of subgroup D. Arch Virol. 1980. 64:225–33.
Article39). Harrison SC. Virology. Looking inside adenovirus. Science. 2010. 329:1026–7.40). Dhurandhar NV., Israel BA., Kolesar JM., Mayhew G., Cook ME., Atkinson RL. Transmissibility of adenovirus-induced adiposity in a chicken model. Int J Obes Relat Metab Disord. 2001. 25:990–6.
Article41). Krishnapuram R., Kirk-Ballard H., Zuberi A., Dhurandhar NV. Infectivity period of mice inoculated with human adenoviruses. Lab Anim. 2011. 45:103–8.
Article42). Dhurandhar NV., Whigham LD., Abbott DH., Schultz-Darken NJ., Israel BA., Bradley SM, et al. Human adenovirus Ad-36 promotes weight gain in male rhesus and marmoset monkeys. J Nutr. 2002. 132:3155–60.
Article43). Atkinson RL., Dhurandhar NV., Allison DB., Bowen RL., Israel BA., Albu JB, et al. Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int J Obes. 2005. 29:281–6.
Article44). Vangipuram SD., Yu M., Tian J., Stanhope KL., Pasarica M., Havel PJ, et al. Adipogenic human adenovirus-36 reduces leptin expression and secretion and increases glucose uptake by fat cells. Int J Obes. 2007. 31:87–96.
Article45). Whigham LD., Israel BA., Atkinson RL. Adipogenic potential of multiple human adenoviruses in vivo and in vitro in animals. Am J Physiol Regul Integr Comp Physiol. 2006. 290:R190–4.46). Vasilakopoulou A., le Roux CW. Could a virus contribute to weight gain? Int J Obes. 2007. 31:1350–6.
Article47). Yao MZ., Gu JF., Wang JH., Sun LY., Liu H., Liu XY. Adenovirus-mediated interleukin-2 gene therapy of nociception. Gene Ther. 2003. 10:1392–9.
Article48). So PW., Herlihy AH., Bell JD. Adiposity induced by adenovirus 5 inoculation. Int J Obes. 2005. 29:603–6.
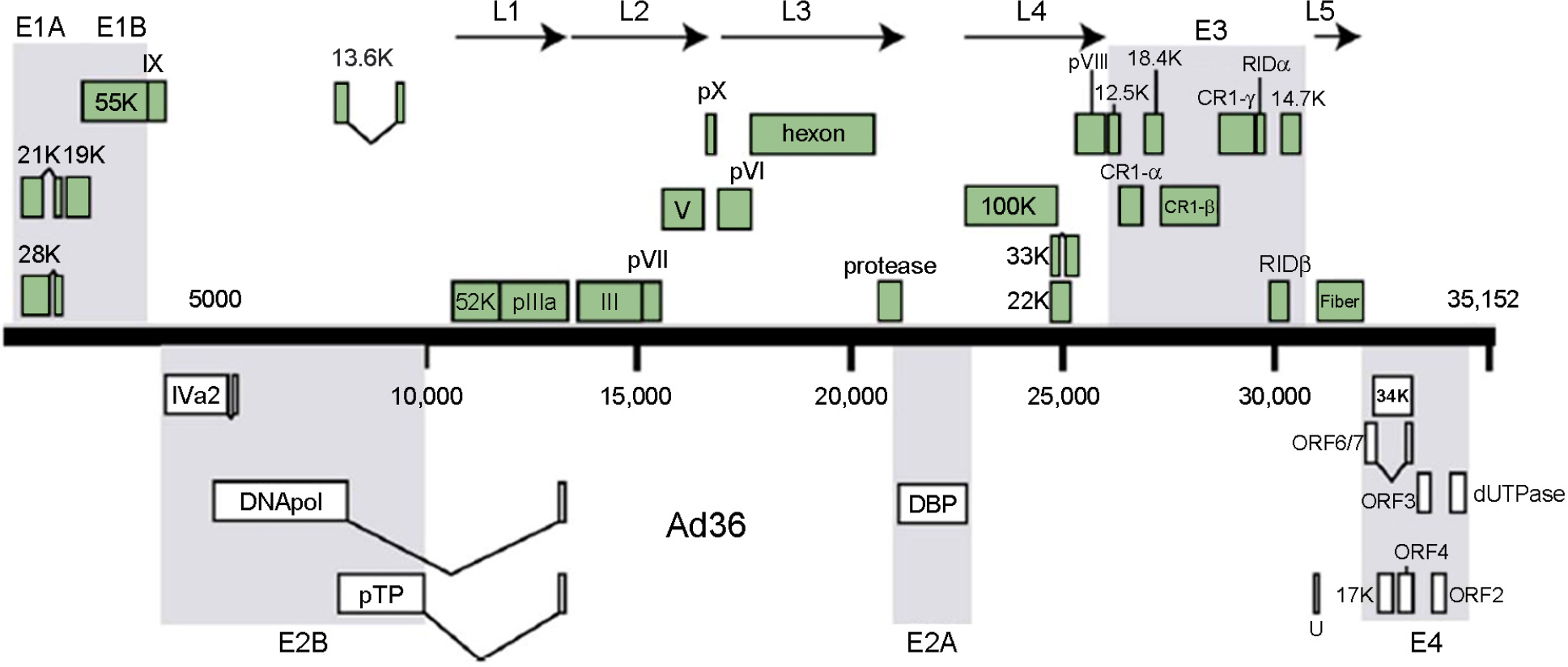
Article49). Arnold J., Janoska M., Kajon AE., Metzgar D., Hudson NR., Torres S, et al. Genomic characterization of human adenovirus 36, a putative obesity agent. Virus Res. 2010. 149:152–61.
Article50). Goossens VJ., deJager SA., Grauls GE., Gielen M., Vlietinck RF., Derom CA, et al. Lack of evidence for the role of human adenovirus-36 in obesity in a European cohort. Obesity. 2011. 19:220–1.
Article51). Broderick MP., Hansen CJ., Irvine M., Metzgar D., Campbell K., Baker C, et al. Adenovirus 36 seropositivity is strongly associated with race and gender, but not obesity, among US military personnel. Int J Obes. 2010. 34:302–8.
Article52). Dhurandhar NV., Israel BA., Kolesar JM., Mayhew GF., Cook ME., Atkinson RL. Increased adiposity in animals due to a human virus. Int J Obes Relat Metab Disord. 2000. 24:989–96.
Article53). Pasarica M., Shin AC., Yu M., Ou Yang HM., Rathod M., Jen KL, et al. Human adenovirus 36 induces adiposity, increases insulin sensitivity, and alters hypothalamic monoamines in rats. Obesity. 2006. 14:1905–13.
Article54). Gabbert C., Donohue M., Arnold J., Schwimmer JB. Adenovirus 36 and obesity in children and adolescents. Pediatrics. 2010. 126:721–6.
Article55). Trovato GM., Martines GF., Garozzo A., Tonzuso A., Timpanaro R., Pirri C, et al. Ad36 adipogenic adeno-virus in human non-alcoholic fatty liver disease. Liver Int. 2010. 30:184–90.
Article56). Na HN., Hong YM., Kim J., Kim HK., Jo I., Nam JH. Association between human adenovirus-36 and lipid disorders in Korean schoolchildren. Int J Obes. 2010. 34:89–93.
Article57). Atkinson RL., Lee I., Shin HJ., He J. Human adenovirus-36 antibody status is associated with obesity in children. Int J Pediatr Obes. 2010. 5:157–60.
Article58). Rogers PM., Fusinski KA., Rathod MA., Loiler SA., Pasarica M., Shaw MK, et al. Human adenovirus Ad-36 induces adipogenesis via its E4 orf-1 gene. Int J Obes. 2008. 32:397–406.
Article59). Pasarica M., Mashtalir N., McAllister EJ., Kilroy GE., Koska J., Permana P, et al. Adipogenic human adeno-virus Ad-36 induces commitment, differentiation, and lipid accumulation in human adipose-derived stem cells. Stem Cells. 2008. 26:969–78.
Article60). Wang ZQ., Cefalu WT., Zhang XH., Yu Y., Qin J., Son L, et al. Human adenovirus type 36 enhances glucose uptake in diabetic and nondiabetic human skeletal muscle cells independent of insulin signaling. Diabetes. 2008. 57:1805–13.
Article61). Laybutt DR., Thompson AL., Cooney GJ., Kraegen EW. Selective chronic regulation of GLUT1 and GLUT4 content by insulin, glucose, and lipid in rat cardiac muscle in vivo. Am J Physiol. 1997. 273:H1309–16.62). Bertacca A., Ciccarone A., Cecchetti P., Vianello B., Laurenza I., Maffei M, et al. Continually high insulin levels impair Akt phosphorylation and glucose transport in human myoblasts. Metabolism. 2005. 54:1687–93.
Article63). Catalán V., Gómez-Ambrosi J., Ramirez B., Rotellar F., Pastor C., Silva C, et al. Proinflammatory cytokines in obesity: impact of type 2 diabetes mellitus and gastric bypass. Obes Surg. 2007. 17:1464–74.
Article64). Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010. 17:332–41.
Article65). Rizvi AA. Cytokine biomarkers, endothelial inflammation, and atherosclerosis in the metabolic syndrome: emerging concepts. Am J Med Sci. 2009. 338:310–8.
Article66). Suga H., Eto H., Aoi N., Kato H., Araki J., Doi K, et al. Adipose tissue remodeling under ischemia: death of adipocytes and activation of stem/progenitor cells. Plast Reconstr Surg. 2010. 126:1911–23.
Article67). Zivkovic AM., German JB., Sanyal AJ. Comparative review of diets for the metabolic syndrome: implications for nonalcoholic fatty liver disease. Am J Clin Nutr. 2007. 86:285–300.
Article68). Nieto-Vazquez I., Fernández-Veledo S., Krämer DK., Vila-Bedmar R., Garcia-Guerra L., Lorenzo M. Insulin resistance associated to obesity: the link TNF-alpha. Arch Physiol Biochem. 2008. 114:183–94.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hepatocellular carcinoma surveillance after sustained virological response in chronic hepatitis C: Editorial on “Non-invasive prediction of post-sustained virological response hepatocellular carcinoma in hepatitis C virus: A systematic review and meta-analysis”
- A Simulation Study on Microbiological Evaluation of Kimbap Manufacturing Process in Summer and Winter
- Durability of antiviral therapy for chronic hepatitis C after achieving sustained virological response
- The Yesterday, Today, and Tomorrow of Pathogen-induced Obesity
- A treatment of drug resistant denture stomatitis by microbiological analysis and adjuvant therapy: a case report