Immune Netw.
2015 Jun;15(3):125-134. 10.4110/in.2015.15.3.125.
Attenuation of Hepatic Graft-versus-host Disease in Allogeneic Recipients of MyD88-deficient Donor Bone Marrow
- Affiliations
-
- 1Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea. ckmin@catholic.ac.kr
- 2Department of Pathology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea.
- 3Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul 110-799, Korea.
- KMID: 2168038
- DOI: http://doi.org/10.4110/in.2015.15.3.125
Abstract
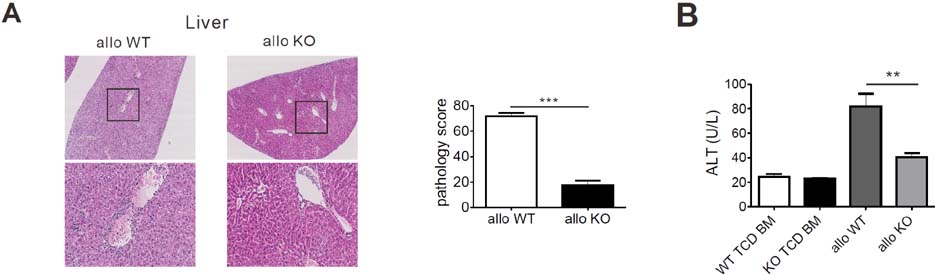
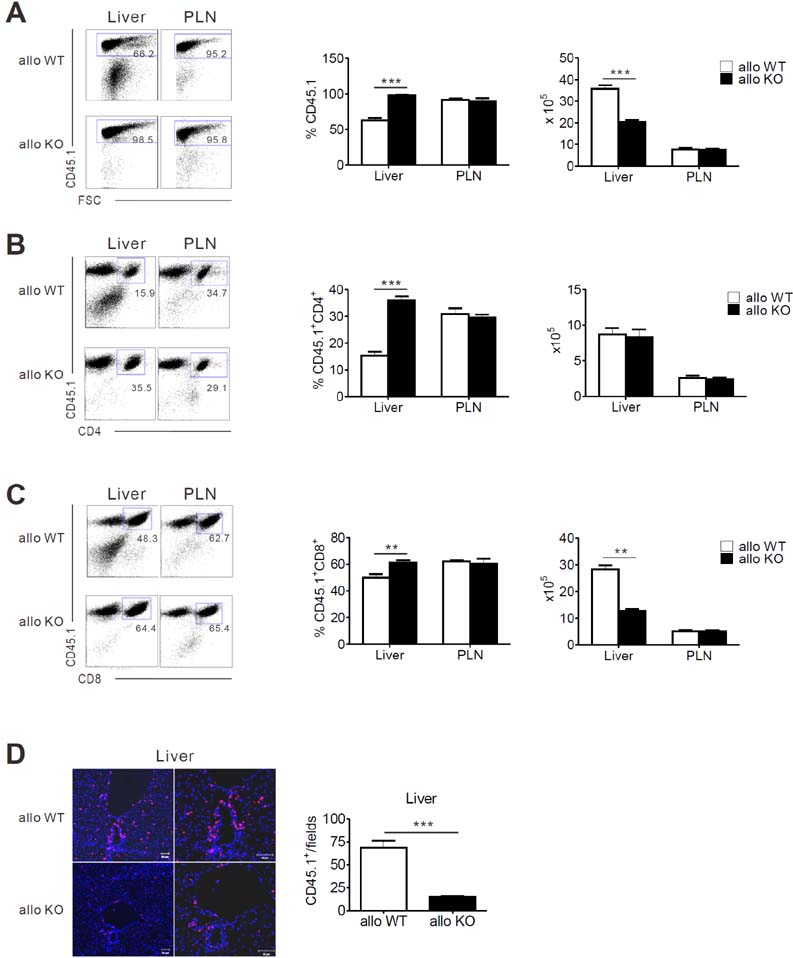
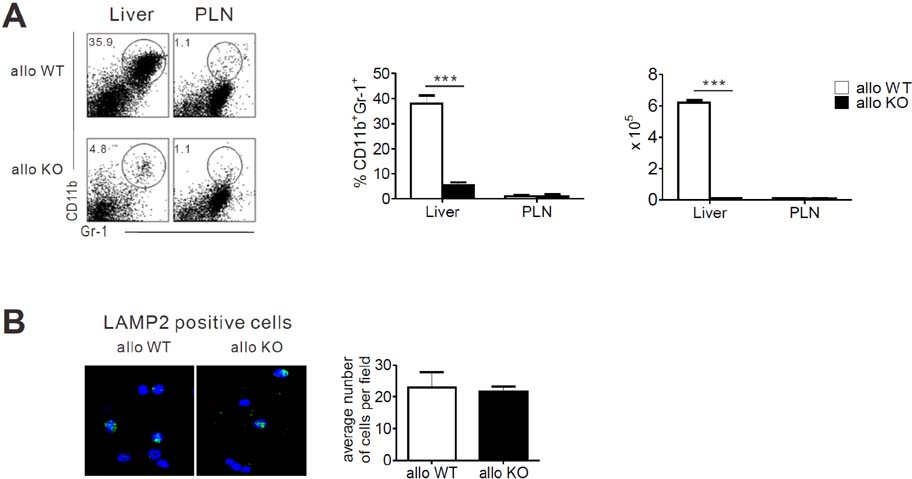
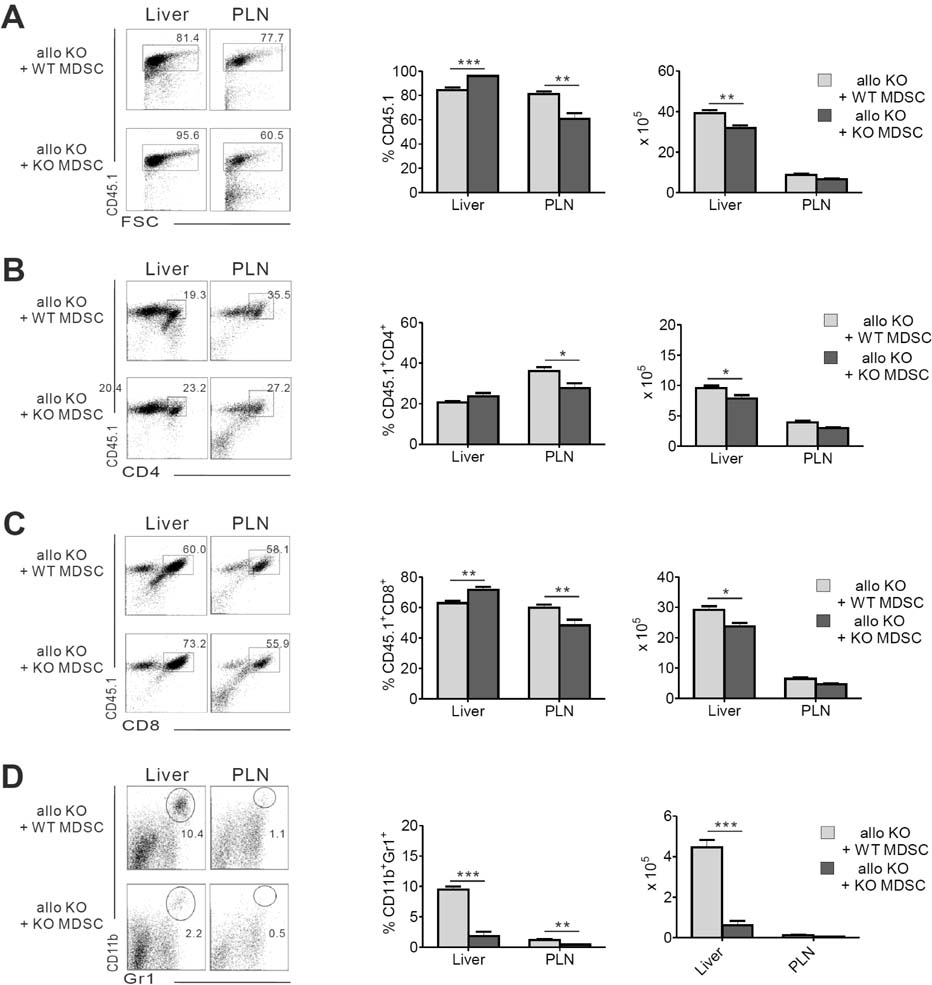
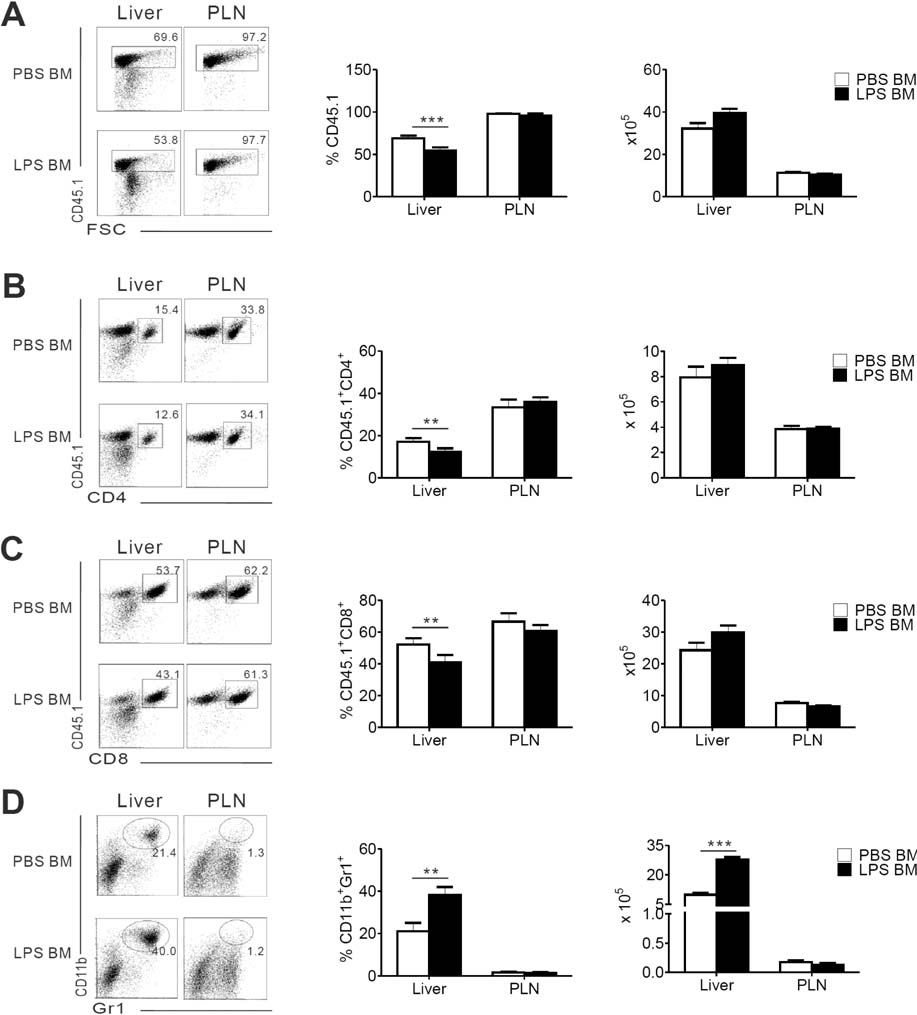
- Acute graft-versus-host-disease (GVHD) is characterized by selective damage to the liver, the skin, and the gastrointestinal tract. Following allogeneic hematopoietic stem cell transplantation, donor bone marrow (BM) cells repopulate the immune system of the recipient. We previously demonstrated that the acute intestinal GVHD (iGVHD) mortality rate was higher in MyD88-deficient BM recipients than that in the control BM recipients. In the present study, the role of MyD88 (expressed by donor BM) in the pathophysiology of hepatic GVHD (hGVHD) was examined. Unlike iGVHD, transplantation with MyD88-deficient T-cell depleted (TCD) BM attenuated hGVHD severity and was associated with low infiltration of T cells into the liver of the recipients. Moreover, GVHD hosts, transplanted with MyD88-deficient TCD BM, exhibited markedly reduced expansion of CD11b(+)Gr-1(+) myeloid-derived suppressor cells (MDSC) in the liver. Adoptive injection of the MDSC from wild type mice, but not MyD88-deficient mice, enhanced hepatic T cell infiltration in the MyD88-deficient TCD BM recipients. Pre-treatment of BM donors with LPS increased MDSC levels in the liver of allogeneic wild type BM recipients. In conclusion, hGVHD and iGVHD may occur through various mechanisms based on the presence of MyD88 in the non-T cell compartment of the allograft.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
TLR/MyD88-mediated Innate Immunity in Intestinal Graft-versus-Host Disease
Young-Kwan Lee, Myungsoo Kang, Eun Young Choi
Immune Netw. 2017;17(3):144-151. doi: 10.4110/in.2017.17.3.144.
Reference
-
1. Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997; 90:3204–3213.
Article2. Caron G, Duluc D, Fremaux I, Jeannin P, David C, Gascan H, Delneste Y. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J Immunol. 2005; 175:1551–1557.
Article3. Heimesaat MM, Nogai A, Bereswill S, Plickert R, Fischer A, Loddenkemper C, Steinhoff U, Tchaptchet S, Thiel E, Freudenberg MA, Gobel UB, Uharek L. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010; 59:1079–1087.
Article4. Quigley M, Martinez J, Huang X, Yang Y. A critical role for direct TLR2-MyD88 signaling in CD8 T-cell clonal expansion and memory formation following vaccinia viral infection. Blood. 2009; 113:2256–2264.
Article5. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004; 118:229–241.
Article6. Szabo G, Dolganiuc A, Mandrekar P. Pattern recognition receptors: a contemporary view on liver diseases. Hepatology. 2006; 44:287–298.
Article7. Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999; 11:115–122.
Article8. Min CK, Maeda Y, Lowler K, Liu C, Clouthier S, Lofthus D, Weisiger E, Ferrara JL, Reddy P. Paradoxical effects of interleukin-18 on the severity of acute graft-versus-host disease mediated by CD4+ and CD8+ T-cell subsets after experimental allogeneic bone marrow transplantation. Blood. 2004; 104:3393–3399.
Article9. Comer GM, Ramey WG, Kotler DP, Holt PR. Isolation of intestinal mononuclear cells from colonoscopic biopsies for immunofluorescence analysis by flow cytometry. Dig Dis Sci. 1986; 31:151–156.
Article10. De Wilde V, Van RN, Hill M, Lebrun JF, Lemaitre P, Lhomme F, Kubjak C, Vokaer B, Oldenhove G, Charbonnier LM, Cuturi MC, Goldman M, Le MA. Endotoxin-induced myeloid-derived suppressor cells inhibit alloimmune responses via heme oxygenase-1. Am J Transplant. 2009; 9:2034–2047.
Article11. Matte CC, Liu J, Cormier J, Anderson BE, Athanasiadis I, Jain D, McNiff J, Shlomchik WD. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004; 10:987–992.
Article12. Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002; 168:554–561.
Article13. Hritz I, Velayudham A, Dolganiuc A, Kodys K, Mandrekar P, Kurt-Jones E, Szabo G. Bone marrow-derived immune cells mediate sensitization to liver injury in a myeloid differentiation factor 88-dependent fashion. Hepatology. 2008; 48:1342–1347.
Article14. Shulman HM, Sharma P, Amos D, Fenster LF, McDonald GB. A coded histologic study of hepatic graft-versus-host disease after human bone marrow transplantation. Hepatology. 1988; 8:463–470.
Article15. Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007; 204:1463–1474.
Article16. Wang D, Yu Y, Haarberg K, Fu J, Kaosaard K, Nagaraj S, Anasetti C, Gabrilovich D, Yu XZ. Dynamic change and impact of myeloid-derived suppressor cells in allogeneic bone marrow transplantation in mice. Biol Blood Marrow Transplant. 2013; 19:692–702.
Article17. Ghansah T, Paraiso KH, Highfill S, Desponts C, May S, McIntosh JK, Wang JW, Ninos J, Brayer J, Cheng F, Sotomayor E, Kerr WG. Expansion of myeloid suppressor cells in SHIP-deficient mice represses allogeneic T cell responses. J Immunol. 2004; 173:7324–7330.
Article18. Zhou Z, French DL, Ma G, Eisenstein S, Chen Y, Divino CM, Keller G, Chen SH, Pan PY. Development and function of myeloid-derived suppressor cells generated from mouse embryonic and hematopoietic stem cells. Stem Cells. 2010; 28:620–632.
Article19. Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, Taylor PA, Panoskaltsis-Mortari A, Serody JS, Munn DH, Tolar J, Ochoa AC, Blazar BR. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010; 116:5738–5747.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recurrent Pneumomediastinum and Subcutaneous Emphysema Complicating Chronic Graft versus Host Disease after Allogeneic Bone Marrow Transplantation
- Skewed Dendritic Cell Differentiation of MyD88-Deficient Donor Bone Marrow Cells, Instead of Massive Expansion as Myeloid-Derived Suppressor Cells, Aggravates GVHD
- A case of transfusion-associated graft-versus-host disease in a preterm infant
- Chronic graft-versus-host disease after allogeneic bone marrow transplantation
- A Case of Acute Follicular Graft versus Host Reaction