Immune Netw.
2011 Oct;11(5):281-287. 10.4110/in.2011.11.5.281.
Nanoliposomes of L-lysine-conjugated poly(aspartic acid) Increase the Generation and Function of Bone Marrow-derived Dendritic Cells
- Affiliations
-
- 1College of Pharmacy, Chungbuk National University, Cheongju 361-763, Korea. cklee@chungbuk.ac.kr
- 2H&A PharmaChem, R&D Center, Bucheon 421-808, Korea.
- 3Cosmeca, R&D Center, Eumseung 869-821, Korea.
- KMID: 2168000
- DOI: http://doi.org/10.4110/in.2011.11.5.281
Abstract
- BACKGROUND
Biodegradable polymers have increasingly been recognized for various biological applications in recent years. Here we examined the immunostimulatory activities of the novel poly(aspartic acid) conjugated with L-lysine (PLA).
METHODS
PLA was synthesized by conjugating L-lysine to aspartic acid polymer. PLA-nanoliposomes (PLA-NLs) were prepared from PLA using a microfluidizer. The immunostimulatory activities of PLA-NLs were examined in mouse bone marrow-derived dendritic cells (BM-DCs).
RESULTS
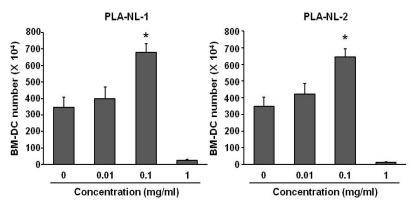
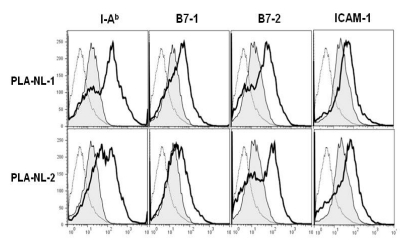
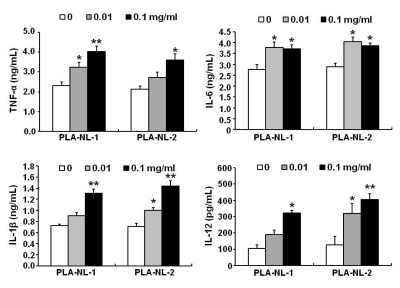
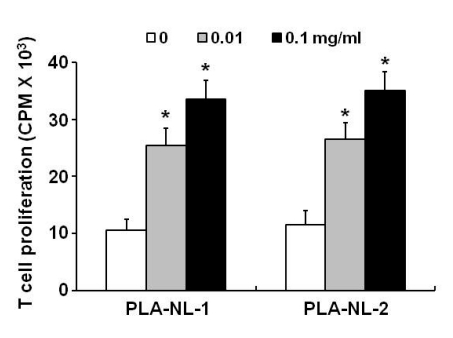
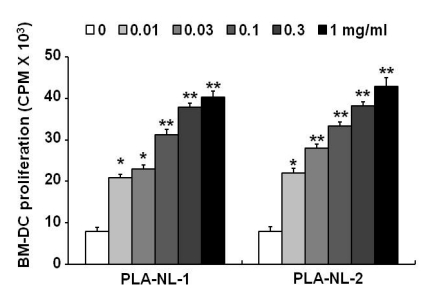
PLA-NLs increased the number of BM-DCs when added to cultures of GM-CSF-induced DC generation on day 4 after the initiation of cultures. Examination of the phenotypic properties showed that BM-DCs generated in the presence of PLA-NLs are more mature in terms of the expression of MHC class II molecules and major co-stimulatory molecules than BM-DCs generated in the absence of PLA-NLs. In addition, the BM-DCs exhibited enhanced capability to produce cytokines, such as IL-6, IL-12, TNF-alpha and IL-1beta. Allogeneic mixed lymphocyte reactions also confirmed that the BMDCs were more stimulatory on allogeneic T cells. PLA- NL also induced further growth of immature BM-DCs that were harvested on day 8.
CONCLUSION
These results show that PLA-NLs induce the generation and functional activities of BM-DCs, and suggest that PLA-NLs could be immunostimulating agents that target DCs.
MeSH Terms
Figure
Reference
-
1. Min SK, Kim SH, Kim JH. Preparation and swelling behavior of biodegradable poly(aspartic acid)-based hydrogel. J Ind Eng Chem. 2000; 6:276–279.2. Goddard JM, Hotchkiss JH. Polymer surface modification for the attachment of bioactive compounds. Progress in Polymer Science. 2007; 32:698–725.
Article3. Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008; 25:1815–1821. PMID: 18373181.
Article4. Elamanchili P, Diwan M, Cao M, Samuel J. Characterization of poly(D,L-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine. 2004; 22:2406–2412. PMID: 15193402.
Article5. Akagi T, Shima F, Akashi M. Intracellular degradation and distribution of protein-encapsulated amphiphilic poly(amino acid) nanoparticles. Biomaterials. 2011; 32:4959–4967. PMID: 21482432.
Article6. Foged C, Sundblad A, Hovgaard L. Targeting vaccines to dendritic cells. Pharm Res. 2002; 19:229–238. PMID: 11934227.7. Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003; 55:329–347. PMID: 12628320.
Article8. Matsusaki M, Hiwatari K, Higashi M, Kaneko T, Akashi M. Stably-dispersed and surface-functional bionanoparticles prepared by self-assembling amphipaethic polymers of hydrophilic poly(g-glutamic acid) bearing hydrophobic amino acids. Chemistry Letters. 2004; 33:398–399.9. Akagi T, Wang X, Uto T, Baba M, Akashi M. Protein direct delivery to dendritic cells using nanoparticles based on amphiphilic poly(amino acid) derivatives. Biomaterials. 2007; 28:3427–3436. PMID: 17482261.
Article10. Giammona G, Pitarresi G, Tomarchio V, Dispenza C, Spadaro G. Synthesis and characterization of water-swellable α,β-polyasparthydrazide derivatives. II. Hydrogels at low crosslinking degree as potential systems for anticancer drug release. Colloid and Polymer Science. 1995; 273:559–564.11. Nakato T, Yoshitake M, Matsubara K, Tomida M, Kakuchi T. Relationships between structure and properties of poly(aspartic acid)s. Macromolecules. 1998; 31:2107–2113.
Article12. Nakato T, Kusuno A, Kakuchi T. Synthesis of poly(succinimide) by bulk polycondensation of L-aspartic acid with an acid catalyst. Journal of Polymer Science Part A: Polymer Chemistry. 2000; 38:117–122.13. Matsubara K, Nakato T, Tomida M. 1H and 13C NMR characterization of poly(succinimide) prepared by thermal polycondensation of l-aspartic acid. Macromolecules. 1997; 30:2305–2312.14. Horgan A, Saunders B, Vincent B, Heenan RK. Poly(butyl methacrylate-g-methoxypoly(ethylene glycol)) and poly (methyl methacrylate-g-methoxypoly(ethylene glycol)) graft copolymers: preparation and aqueous solution properties. J Colloid Interface Sci. 2003; 262:548–559. PMID: 16256637.15. Zhu G. Micellization of polystyrene-graft-poly(ethylene oxide) and its mixtures with polystyrene homopolymer in ethanol. European Polymer Journal. 2005; 41:2671–2677.
Article16. Li P, Yin YL, Li D, Kim SW, Wu G. Amino acids and immune function. Br J Nutr. 2007; 98:237–252. PMID: 17403271.
Article17. Kidd MT, Kerr BJ, Anthony NB. Dietary interactions between lysine and threonine in broilers. Poult Sci. 1997; 76:608–614. PMID: 9106889.
Article18. Konashi S, Takahashi K, Akiba Y. Effects of dietary essential amino acid deficiencies on immunological variables in broiler chickens. Br J Nutr. 2000; 83:449–456. PMID: 10858703.19. Yang SR, Lee HJ, Kim JD. Histidine-conjugated poly(amino acid) derivatives for the novel endosomolytic delivery carrier of doxorubicin. J Control Release. 2006; 114:60–68. PMID: 16828916.
Article20. Xu Q, An L, Yu M, Wang S. Design and synthesis of a new conjugated polyelectrolyte as a reversible ph sensor. Macromolecular Rapid Communications. 2008; 29:390–395.
Article21. Jeong JH, Park TG. Poly(L-lysine)-g-poly(D,L-lactic-co-glycolic acid) micelles for low cytotoxic biodegradable gene delivery carriers. J Control Release. 2002; 82:159–166. PMID: 12106986.
Article22. Stone WL, Mukherjee S, Smith M, Das SK. Therapeutic uses of antioxidant liposomes. Methods Mol Biol. 2002; 199:145–161. PMID: 12094566.
Article23. Stone WL, Smith M. Therapeutic uses of antioxidant liposomes. Mol Biotechnol. 2004; 27:217–230. PMID: 15247495.
Article24. Chen CH, Liu DZ, Fang HW, Liang HJ, Yang TS, Lin SY. Evaluation of multi-target and single-target liposomal drugs for the treatment of gastric cancer. Biosci Biotechnol Biochem. 2008; 72:1586–1594. PMID: 18540096.
Article25. Koike M, Ishino K, Kohno Y, Tachikawa T, Kartasova T, Kuroki T, Huh N. DMSO induces apoptosis in SV40-transformed human keratinocytes, but not in normal keratinocytes. Cancer Lett. 1996; 108:185–193. PMID: 8973593.
Article26. Paromov V, Kumari S, Brannon M, Kanaparthy NS, Yang H, Smith MG, Stone WL. Protective effect of liposome-encapsulated glutathione in a human epidermal model exposed to a mustard gas analog. J Toxicol. 2011; 2011:109516. PMID: 21776256.
Article27. Kim SI, Min SK, Kim JH. Synthesis and characterization of novel amino acid-conjugated poly(aspartic acid) derivatives. Bull Korean Chem Soc. 2008; 29:1887–1892.28. Lee JK, Lee MK, Yun YP, Kim Y, Kim JS, Kim YS, Kim K, Han SS, Lee CK. Acemannan purified from Aloe vera induces phenotypic and functional maturation of immature dendritic cells. Int Immunopharmacol. 2001; 1:1275–1284. PMID: 11460308.
Article29. Lee YR, Lee YH, Im SA, Kim K, Lee CK. Formulation and characterization of antigen-loaded plga nanoparticles for efficient cross-priming of the antigen. Immune Netw. 2011; 11:163–168. PMID: 21860609.
Article30. Im SA, Lee YR, Lee YH, Oh ST, Gerelchuluun T, Kim BH, Kim Y, Yun YP, Song S, Lee CK. Synergistic activation of monocytes by polysaccharides isolated from Salicornia herbacea and interferon-gamma. J Ethnopharmacol. 2007; 111:365–370. PMID: 17204386.31. Lee YH, Lee YR, Kim KH, Im SA, Song S, Lee MK, Kim Y, Hong JT, Kim K, Lee CK. Baccatin III, a synthetic precursor of taxol, enhances MHC-restricted antigen presentation in dendritic cells. Int Immunopharmacol. 2011; 11:985–991. PMID: 21354357.
Article32. Talmor M, Mirza A, Turley S, Mellman I, Hoffman LA, Steinman RM. Generation or large numbers of immature and mature dendritic cells from rat bone marrow cultures. Eur J Immunol. 1998; 28:811–817. PMID: 9541575.
Article33. Mora JR. Homing imprinting and immunomodulation in the gut: role of dendritic cells and retinoids. Inflamm Bowel Dis. 2008; 14:275–289. PMID: 17924560.
Article34. Cai Z, Brunmark AB, Luxembourg AT, Garcia KC, Degano M, Teyton L, Wilson I, Peterson PA, Sprent J, Jackson MR. Probing the activation requirements for naive CD8+ T cells with Drosophila cell transfectants as antigen presenting cells. Immunol Rev. 1998; 165:249–265. PMID: 9850865.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Defects in the differentiation and function of bone marrow-derived dendritic cells in non-obese diabetic mice
- Generation and Characterization of Alloenic Radiation Bone Marrow Chimera
- In vitro effects of monophosphoryl lipid A and Poly I:C combination on equine cells
- Retained Endocytic Activity in Bone Marrow-derived Dendritic Cells Expressing Surface MHC Class II Molecules
- The Cytotoxicity of Poly - L - lysine in Different Concentration to Preadipocytes Harvested from Living Rats