Immune Netw.
2009 Feb;9(1):27-33. 10.4110/in.2009.9.1.27.
Production of TGF-beta1 as a Mechanism for Defective Antigen-presenting Cell Function of Macrophages Generated in vitro with M-CSF
- Affiliations
-
- 1School of Science Education (Biology), Chungbuk National University, Cheongju, Korea.
- 2College of Pharmacy, Chungbuk National University, Cheongju, Korea. cklee@chungbuk.ac.kr
- 3College of Pharmacy, Sahmyook University, Seoul, Korea.
- KMID: 2167994
- DOI: http://doi.org/10.4110/in.2009.9.1.27
Abstract
-
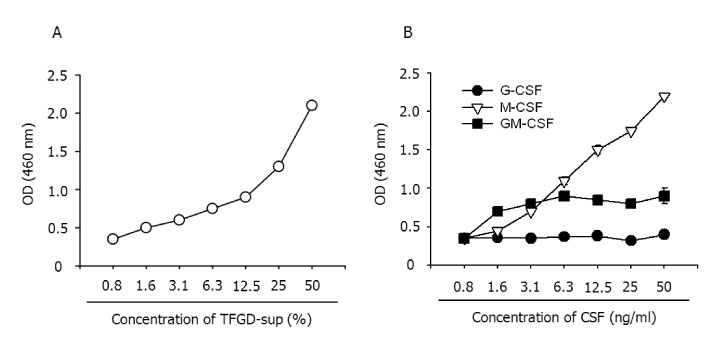
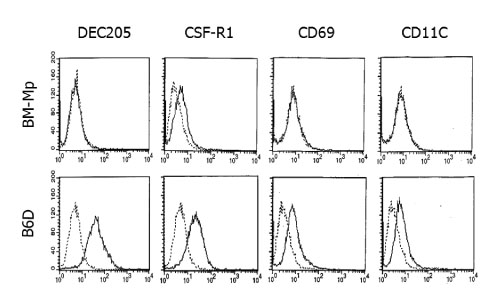
BACKGROUND: Macrophages generated in vitro using macrophage-colony stimulating factor (M-CSF) and interleukin (IL)-6 from bone marrow cells (BM-Mp) are defective in antigen presenting cell (APC) function as shown by their ability to induce the proliferation of anti-CD3 mAb-primed syngeneic T cells. However, they do express major histocompatibility (MHC) class I and II molecules, accessory molecules and intracellular adhesion molecules. Here we demonstrate that the defective APC function of macrophages is mainly due to production of TGF-beta1 by BM-Mp.
METHODS
Microarray analysis showed that TGF-beta1 was highly expressed in BM-Mp, compared to a macrophage cell line, B6D, which exerted efficient APC function. Production of TGF-beta1 by BM-Mp was confirmed by neutralization experiments of TGF-beta1 as well as by real time-polymerase chain reaction (PCR).
RESULTS
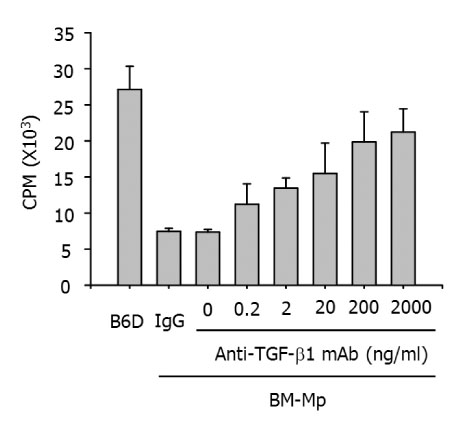
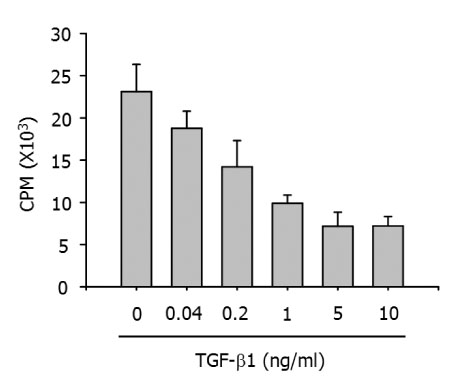
Addition of anti-TGF-beta1 monoclonal antibody to cultures of BM-Mp and anti-CD3 mAb-primed syngeneic T cells efficiently induced the proliferation of syngeneic T cells. Conversely, the APC function of B6D cells was almost completely suppressed by addition of TGF-beta1. Quantitative real time-PCR analysis also confirmed the enhanced expression of TGF-beta1 in BM-Mp.
CONCLUSION
The defective APC function of macrophages generated in vitro with M-CSF and IL-6 was mainly due to the production of TGF-beta1 by macrophages.
Keyword
MeSH Terms
Figure
Reference
-
1. Jansen JH, Kluin-Nelemans JC, van Damme J, Weintjens GJ, Willemze R, Fibbe WE. Interleukin 6 is a permissive factor for monocytic colony formation by human hematopoietic cells. J Exp Med. 1992. 175:1151–1154.
Article2. Khalili H, Deshpande R, Chang MY. The defective antigen-presenting activity of murine fetal macrophage cell lines. Immunology. 1997. 92:487–493.
Article3. Smith W, Feldmann M, Londei M. Human macrophages induced in vitro by macrophage colony-stimulating factor are deficient in IL-12 production. Eur J Immunol. 1998. 28:2498–2507.
Article4. Lee CK, Kim JK, Kim Y, Lee MK, Kim K, Kang JK, Hofmeister R, Durum SK, Han SS. Generation of macrophages from early T progenitors in vitro. J Immunol. 2001. 166:5964–5969.
Article5. Holt PG, Oliver J, Bilyk N, McMenamin C, McMenamin PG, Kraal G, Thepen T. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med. 1993. 177:397–407.
Article6. Miyazaki T, Suzuki G, Yamamura K. The role of macrophages in antigen presentation and T cell tolerance. Int Immunol. 1993. 5:1023–1033.
Article7. Poulter LW, Janossy G, Power C, Sreenan S, Burke C. Immunological/physiological relationships in asthma: potential regulation by lung macrophages. Immunol Today. 1994. 15:258–261.
Article8. Munn DH, Pressey J, Beall AC, Hudes R, Alderson MR. Selective activation-induced apoptosis of peripheral T cells imposed by macrophages. A potential mechanism of antigen-specific peripheral lymphocyte deletion. J Immunol. 1996. 156:523–532.9. Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994. 372:100–103.
Article10. Lee JK, Kim JK, Lee YR, Kim HS, Im SA, Kim K, Lee CK. Exposure to chemokines during maturation modulates antigen presenting cell function of mature macrophages. Cell Immunol. 2005. 234:1–8.
Article11. Kehrl JH, Roberts AB, Wakefield LM, Jakowlew S, Sporn MB, Fauci AS. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986. 137:3855–3860.12. Ristow HJ. BSC-1 growth inhibitor/type beta transforming growth factor is a strong inhibitor of thymocyte proliferation. Proc Natl Acad Sci U S A. 1986. 83:5531–5533.
Article13. Rook AH, Kehrl JH, Wakefield LM, Roberts AB, Sporn MB, Burlington DB, Lane HC, Fauci AS. Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J Immunol. 1986. 136:3916–3920.14. Kuppner MC, Hamou MF, Bodmer S, Fontana A, de Tribolet N. The glioblastoma-derived T-cell suppressor factor/transforming growth factor beta 2 inhibits the generation of lymphokine-activated killer (LAK) cells. Int J Cancer. 1988. 42:562–567.
Article15. Wahl SM, Hunt DA, Bansal G, McCartney-Francis N, Eliingsworth L, Allen JB. Bacterial cell wall-induced immunosuppression. Role of transforming growth factor beta. J Exp Med. 1988. 168:1403–1417.
Article16. Miki R, Kadota K, Bono H, Mizuno Y, Tomaru Y, Carninci P, Itoh M, Shibata K, Kawai J, Konno H, Watanabe S, Sato K, Tokusumi Y, Kikuchi N, Ishii Y, Hamaguchi Y, Nishizuka I, Goto H, Nitanda H, Satomi S, Yoshiki A, Kusakabe M, DeRisi JL, Eisen MB, Iyer VR, Brown PO, Muramatsu M, Shimada H, Okazaki Y, Hayashizaki Y. Delineating developmental and metabolic pathways in vivo by expression profiling using the RIKEN set of 18,816 full-length enriched mouse cDNA arrays. Proc Natl Acad Sci U S A. 2001. 98:2199–2204.
Article17. Liu W, Saint DA. A new quantitative methods of real time reverse transcription polymerase chain reaction assay based on stimulation of polymerase chain reaction kinetics. Anal Biochem. 2002. 302:52–59.
Article18. Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, Blay JY. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of intereukin-6 and macrophage colony-stimulating factor. Blood. 1998. 92:4778–4791.
Article19. Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997. 9:10–16.
Article20. Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994. 179:1109–1118.
Article21. Wong GG, Temple PA, Leary AC, Witek-Giannotti JS, Yang CY, Ciarletta AB, Chung M, Murtha P, Kriz R, Kaufman RJ, Ferenz CR, Sibley BS, Tunrner KJ, Hewick RM, Clark SC, Yanai N, Yamada M, Saito M, Motoyoshi K, Takaku F. Human CSF-1: molecular cloning and expression of 4-kb cDNA encoding the human urinary protein. Science. 1987. 235:1504–1508.
Article22. Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986. 67:257–267.
Article23. Espevik T, Figari I, Ranges GE, Palladino MA Jr. Transforming growth factor-beta 1 (TGF-beta 1) and recombinant human tumor necrosis factor-alpha reciprocally regulate the generation of lymphokine-activated killer cell activity. Comparison between natural porcine platelet-derived TGF-beta 1 and TGF-beta 2, and recombinant human TGF-beta 1. J Immunol. 1988. 140:2312–2316.24. Ohta M, Greenberger JS, Anklesaria P, Bassols A, Massaqué J. Two forms of transforming growth factor-beta distinguished by multipotential haematopoietic progenitor cells. Nature. 1987. 329:539–541.
Article25. Ranges GE, Figari IS, Espevik T, Palladino MA Jr. Inhibition of cytotoxic T cell development by transforming growth factor beta and reversal by recombinant tumor necrosis factor alpha. J Exp Med. 1987. 166:991–998.
Article26. Czarniecki CW, Chiu HH, Wong GH, McCabe SM, Palladino MA. Transforming growth factor-beta 1 modulates the expression of class II histocompatibility antigens on human cells. J Immunol. 1988. 140:4217–4223.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of TGF-beta1 Protein in Macrophages of Tuberculous Granulomas
- An Experimental Study of the Effect of TGF-beta1 on the Growth and Apoptosis in Y-79 Retinoblastoma Cells
- Production Of Gm-Csf And Tgf-beta 1 In Irradiated Human Gingival Fibroblasts Cultured With Lipopolysaccharide
- Production of Transforming Growth Factor-beta1 in Human Fibroblasts Induced with Bacterial Toxins
- Inhibition of Cell Growth and Suppression of c-myc Gene Expression by Transforming Growth Factor-beta1 in Cervical Carcinoma Cell Lines