Clin Exp Vaccine Res.
2013 Jul;2(2):115-119. 10.7774/cevr.2013.2.2.115.
Long-term immunogenicity of the influenza vaccine at reduced intradermal and full intramuscular doses among healthy young adults
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. heejinmd@medimail.co.kr
- 2Asian Pacific Influenza Institute, Korea University College of Medicine, Seoul, Korea.
- KMID: 2167721
- DOI: http://doi.org/10.7774/cevr.2013.2.2.115
Abstract
- PURPOSE
To prepare for vaccine shortages under an influenza pandemic, several antigen-sparing strategies have been investigated. This study was aimed to evaluate the immunogenicity of influenza vaccine at reduced intradermal and full intramuscular dose.
MATERIALS AND METHODS
We compared the effect of one-fifth and one-half intradermal doses to the full intramuscular dose on immunogenicity in healthy young adults, using a commercial influenza vaccine. A hemagglutination inhibition assay was used to compare the immunogenicity of the vaccination methods.
RESULTS
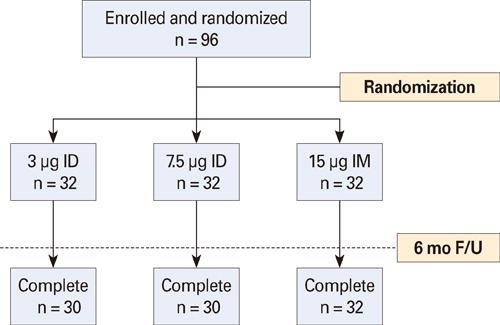
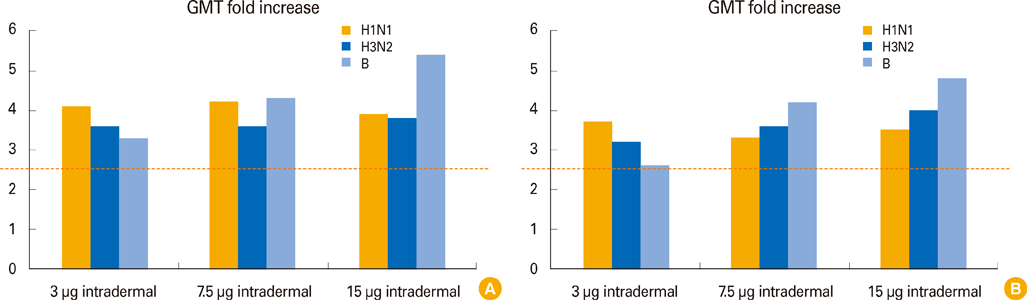
The one-fifth intradermal dose (3 microg hemagglutinin antigen, HA) was given to 30 participants, the one-half intradermal dose (7.5 microg HA) was given to 30, and the full intramuscular dose (15 microg HA) was given to 32. No significant differences among injection routes and dosages were seen for seroprotection rate, seroconversion rate, or geometric mean titer (GMT) fold-increase for A/H1N1, A/H3N2, and B at around 4 weeks from vaccination. Although GMT for influenza B was significantly lower at six months for the one-fifth intradermal vaccination compared to the full-dose intramuscular vaccination (32.8 vs. 63.2, p=0.048), all three groups met the Evaluation of Medicinal Products (EMA) immunogenicity criteria through 1 to 6 months.
CONCLUSION
Intradermal administration of a one-fifth dose of influenza vaccine elicited antibody responses comparable to the intradermal one-half dose and a conventional intramuscular vaccination at 1 month post-vaccination. The immunogenicity of the one-fifth intradermal dose was sufficient to meet the requirement for the EMA criteria at six months after influenza vaccination.
MeSH Terms
Figure
Reference
-
1. Jakob T, Udey MC. Epidermal Langerhans cells: from neurons to nature's adjuvants. Adv Dermatol. 1999; 14:209–258.2. Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004; 351:2295–2301.
Article3. La Montagne JR, Fauci AS. Intradermal influenza vaccination: can less be more? N Engl J Med. 2004; 351:2330–2332.4. Lambert PH, Laurent PE. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine. 2008; 26:3197–3208.
Article5. Nicolas JF, Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines. 2008; 7:1201–1214.
Article6. Cheong HJ, Song JY, Park JW, et al. Humoral and cellular immune responses to influenza vaccine in patients with advanced cirrhosis. Vaccine. 2006; 24:2417–2422.
Article7. Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond). 1972; 70:767–777.
Article8. Davies JR, Grilli EA. Natural or vaccine-induced antibody as a predictor of immunity in the face of natural challenge with influenza viruses. Epidemiol Infect. 1989; 102:325–333.
Article9. European Committee for Proprietary Medicinal Products. Note for guidance on harmonisation of requirements for influenza vaccines. London: The Europen Agency for the Evaluation of Medicinal Products;1997.10. Koff RS. Immunogenicity of hepatitis B vaccines: implications of immune memory. Vaccine. 2002; 20:3695–3701.
Article11. Young F, Marra F. A systematic review of intradermal influenza vaccines. Vaccine. 2011; 29:8788–8801.
Article12. Marra F, Young F, Richardson K, Marra CA. A meta-analysis of intradermal versus intramuscular influenza vaccines: immunogenicity and adverse events. Influenza Other Respi Viruses. 2013; 7:584–603.
Article13. Belshe RB, Newman FK, Cannon J, et al. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004; 351:2286–2294.
Article14. Auewarakul P, Kositanont U, Sornsathapornkul P, Tothong P, Kanyok R, Thongcharoen P. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine. 2007; 25:659–663.
Article15. Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009; 27:454–459.
Article16. Chuaychoo B, Wongsurakiat P, Nana A, Kositanont U, Maranetra KN. The immunogenicity of intradermal influenza vaccination in COPD patients. Vaccine. 2010; 28:4045–4051.
Article17. Belshe RB, Newman FK, Wilkins K, et al. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults. Vaccine. 2007; 25:6755–6763.
Article18. Beran J, Ambrozaitis A, Laiskonis A, et al. Intradermal influenza vaccination of healthy adults using a new microinjection system: a 3-year randomised controlled safety and immunogenicity trial. BMC Med. 2009; 7:13.
Article19. Arnou R, Eavis P, Pardo JR, Ambrozaitis A, Kazek MP, Weber F. Immunogenicity, large scale safety and lot consistency of an intradermal influenza vaccine in adults aged 18-60 years: randomized, controlled, phase III trial. Hum Vaccin. 2010; 6:346–354.
Article20. Chi RC, Rock MT, Neuzil KM. Immunogenicity and safety of intradermal influenza vaccination in healthy older adults. Clin Infect Dis. 2010; 50:1331–1338.
Article21. Leroux-Roels I, Vets E, Freese R, et al. Seasonal influenza vaccine delivered by intradermal microinjection: a randomised controlled safety and immunogenicity trial in adults. Vaccine. 2008; 26:6614–6619.
Article22. Campos-Outcalt D. CDC recommendations expand vaccine indications. J Fam Pract. 2009; 58:146–148.23. Ansaldi F, de Florentiis D, Durando P, Icardi G. Fluzone((R)) Intradermal vaccine: a promising new chance to increase the acceptability of influenza vaccination in adults. Expert Rev Vaccines. 2012; 11:17–25.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunogenicity and Safety of Half Dose Intradermal Injection Compared to Full Dose Intramuscular Injection of Influenza Vaccine in Healthy Adults
- Fluad(R)-MF59(R)-Adjuvanted Influenza Vaccine in Older Adults
- Assessment of Influenza Vaccine Immunogenicity in Immunocompromized Host During 2009 Influenza Season: A Single Institution Experience
- Sleep and vaccine administration time as factors influencing vaccine immunogenicity
- Influenza Vaccine