Cancer Res Treat.
2014 Apr;46(2):148-157.
Impact on Loco-regional Control of Radiochemotherapeutic Sequence and Time to Initiation of Adjuvant Treatment in Stage II/III Rectal Cancer Patients Treated with Postoperative Concurrent Radiochemotherapy
- Affiliations
-
- 1Department of Radiation Oncology, Hallym University Dongtan Sacred Heart Hospital, Hwasung, Korea.
- 2Department of Radiation Oncology, Seoul National University College of Medicine, Seoul, Korea. ekchie93@snu.ac.kr
- 3Department of Radiation Oncology, Samsung Comprehensive Cancer Center, Sungkyunkwan University School of Medicine, Seoul, Korea. ahnyc@skku.edu
- 4Department of Radiation Oncology, Korea University Ansan Hospital, Ansan, Korea.
Abstract
- PURPOSE
This study was designed to evaluate the impact of radiochemotherapeutic sequence and time to initiation of adjuvant treatment on loco-regional control for resected stage II and III rectal cancer.
MATERIALS AND METHODS
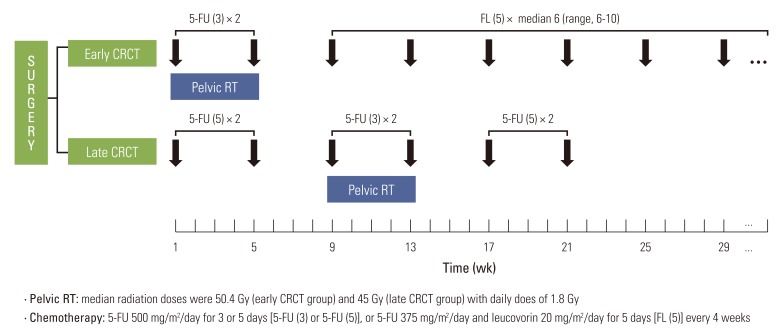
Treatment outcomes for rectal cancer patients from two hospitals with different sequencing strategies regarding adjuvant concurrent radiochemotherapy (CRCT) were compared retrospectively. Pelvic radiotherapy was administered concurrently on the first (early CRCT, n=180) or the third cycle of chemotherapy (late CRCT, n=180). During radiotherapy, two cycles of fluorouracil were provided to patients in both groups. In the early CRCT group, median six cycles of fluorouracil and leucovorin were prescribed during the post-CRCT period. In the late CRCT group, two cycles of fluorouracil were administered in the pre- and post-CRCT periods.
RESULTS
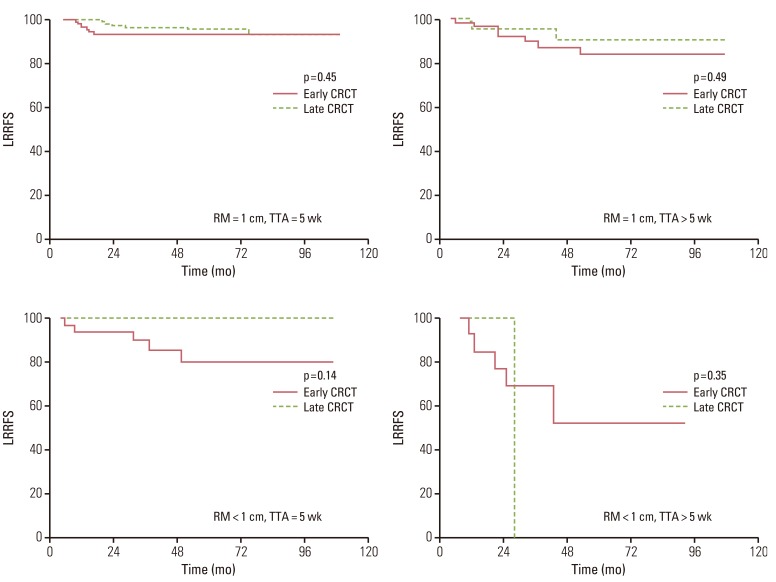
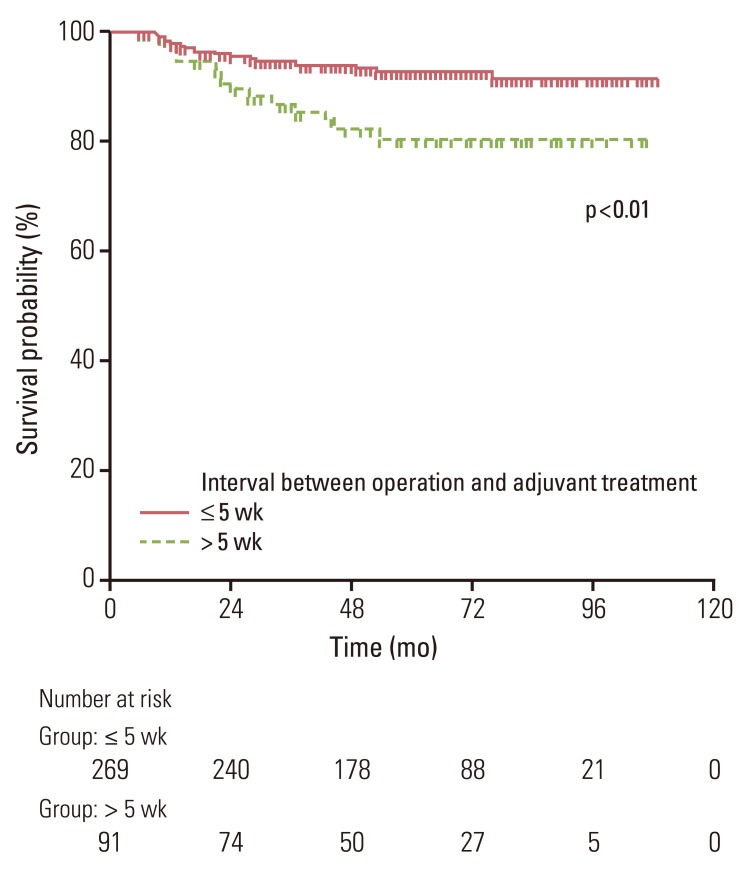
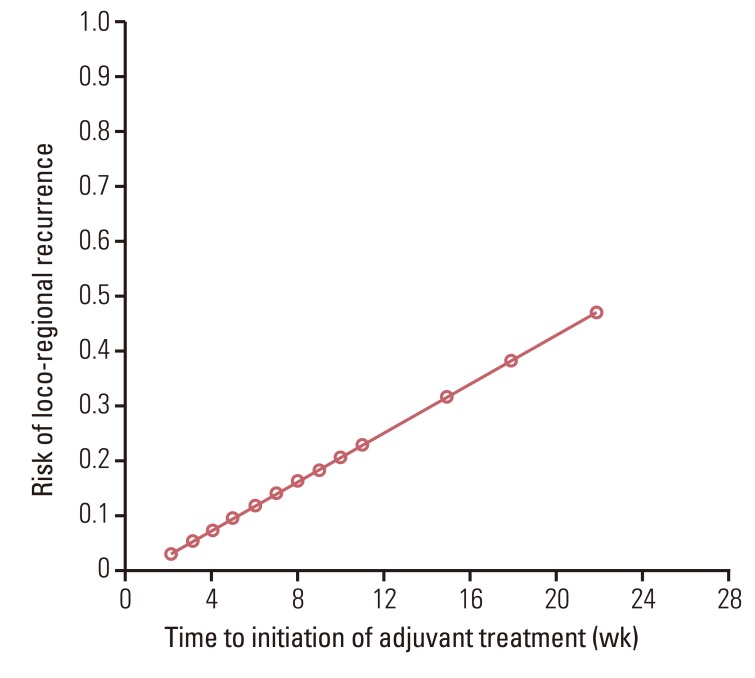
No significant differences in the 5-year loco-regional recurrence-free survival (LRRFS) (92.5% vs. 95.6%, p=0.43) or overall survival and disease-free survival were observed between groups. Patients who began receiving adjuvant treatment later than five weeks after surgery had lower LRRFS than patients who received adjuvant treatment within five weeks following surgery (79% vs. 91%, p<0.01). The risk of loco-regional recurrence increased as the time to initiation of adjuvant treatment was delayed.
CONCLUSION
In the current study, treatment outcomes were not significantly influenced by the sequence of adjuvant treatment but by the delay of adjuvant treatment for more than five weeks. Timely administration of adjuvant treatment is deemed important in achieving loco-regional tumor control for stage II/III rectal cancer patients.
MeSH Terms
Figure
Reference
-
1. McCall JL, Cox MR, Wattchow DA. Analysis of local recurrence rates after surgery alone for rectal cancer. Int J Colorectal Dis. 1995; 10:126–132. PMID: 7561427.
Article2. Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med. 1985; 312:1465–1472. PMID: 2859523.3. Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991; 324:709–715. PMID: 1997835.
Article4. Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004; 351:1731–1740. PMID: 15496622.
Article5. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012; 30:1926–1933. PMID: 22529255.
Article6. Smalley SR, Benedetti JK, Williamson SK, Robertson JM, Estes NC, Maher T, et al. Phase III trial of fluorouracil-based chemotherapy regimens plus radiotherapy in postoperative adjuvant rectal cancer: GI INT 0144. J Clin Oncol. 2006; 24:3542–3547. PMID: 16877719.
Article7. Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K, et al. Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys. 2000; 47:13–47. PMID: 10758303.
Article8. Kim JH, Oh DH, Kang KM, Kim WC, Kim WD, Kim JS, et al. Postoperative radiotherapy in the rectal cancers patterns of care study for the years of 1998~1999. J Korean Soc Ther Radiol Oncol. 2005; 23:22–31.9. Lee JH, Lee JH, Ahn JH, Bahng H, Kim TW, Kang YK, et al. Randomized trial of postoperative adjuvant therapy in stage II and III rectal cancer to define the optimal sequence of chemotherapy and radiotherapy: a preliminary report. J Clin Oncol. 2002; 20:1751–1758. PMID: 11919231.
Article10. Kim TW, Lee JH, Lee JH, Ahn JH, Kang YK, Lee KH, et al. Randomized trial of postoperative adjuvant therapy in Stage II and III rectal cancer to define the optimal sequence of chemotherapy and radiotherapy: 10-year follow-up. Int J Radiat Oncol Biol Phys. 2011; 81:1025–1031. PMID: 20932669.
Article11. Chau I, Norman AR, Cunningham D, Tait D, Ross PJ, Iveson T, et al. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol. 2005; 16:549–557. PMID: 15695501.
Article12. Cheung WY, Neville BA, Earle CC. Etiology of delays in the initiation of adjuvant chemotherapy and their impact on outcomes for Stage II and III rectal cancer. Dis Colon Rectum. 2009; 52:1054–1063. PMID: 19581846.
Article13. Ahmed S, Ahmad I, Zhu T, Arnold FP, Faiz Anan G, Sami A, et al. Early discontinuation but not the timing of adjuvant therapy affects survival of patients with high-risk colorectal cancer: a population-based study. Dis Colon Rectum. 2010; 53:1432–1438. PMID: 20847626.
Article14. Des Guetz G, Nicolas P, Perret GY, Morere JF, Uzzan B. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer. 2010; 46:1049–1055. PMID: 20138505.
Article15. Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011; 305:2335–2342. PMID: 21642686.16. Goldie JH, Coldman AJ. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep. 1979; 63:1727–1733. PMID: 526911.17. Dent OF, Haboubi N, Chapuis PH, Chan C, Lin BP, Wong SK, et al. Assessing the evidence for an association between circumferential tumour clearance and local recurrence after resection of rectal cancer. Colorectal Dis. 2007; 9:112–121. PMID: 17223934.
Article18. Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994; 344:707–711. PMID: 7915774.
Article19. Nagtegaal ID, Marijnen CA, Kranenbarg EK, van de Velde CJ, van Krieken JH. Pathology Review Committee. Cooperative Clinical Investigators. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002; 26:350–357. PMID: 11859207.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Radiation Therapy as an Adjuvant Treatment in Rectal Cancer Management
- Comparing the initiation of adjuvant chemotherapy after robotic and laparoscopic colon cancer surgeries: A case-controlled study with propensity score matching
- Postoperative Adjuvant Chemotherapy and Chemoradiation for Rectal Cancer
- Dose-Response Relationship between Radiation Dose and Loco-regional Control in Patients with Stage II-III Esophageal Cancer Treated with Definitive Chemoradiotherapy
- Postoperative Adjuvant Chemoradiotherapy in Rectal Cancer