Allergy Asthma Immunol Res.
2015 Jul;7(4):367-375. 10.4168/aair.2015.7.4.367.
TMEM16A-Mediated Mucin Secretion in IL-13-Induced Nasal Epithelial Cells From Chronic Rhinosinusitis Patients
- Affiliations
-
- 1Department of Otolaryngology, Head and Neck Surgery, Beijing Tongren Hospital, Capital Medical University, Beijing, China. dr.luozhang@gmail.com
- 2Key Laboratory of Otolaryngology, Head and Neck Surgery, Ministry of Education, Beijing Institute of Otolaryngology, Beijing, China.
- 3Upper Airways Research Laboratory, Department of Oto-Rhino-Laryngology, Ghent University Hospital, Ghent, Belgium.
- KMID: 2166684
- DOI: http://doi.org/10.4168/aair.2015.7.4.367
Abstract
- PURPOSE
Chronic rhinosinusitis with nasal polyps (CRSwNP), a mainly Th2 cytokine-mediated disease, often involves mucus secretion. Recent evidence suggests that transmembrane protein 16A (TMEM16A), a calcium-activated Cl- channel (CaCC), can regulate mucus secretion from airway epithelium by transepithelial electrolyte transport and hydration. However, the role of TMEM16A in mucin production/secretion in the airway epithelium is not clear. This study was conducted to determine the role of TMEM16A in mediating mucin secretion in human nasal polyp epithelial cells (HNPECs) induced by IL-13.
METHODS
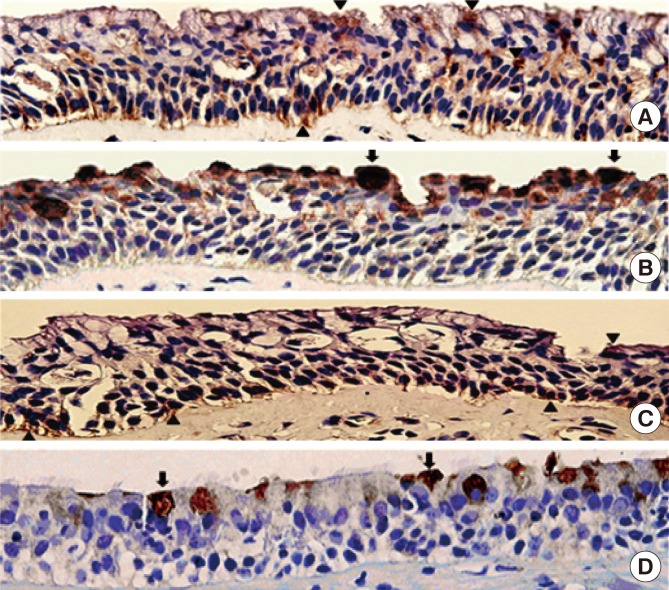
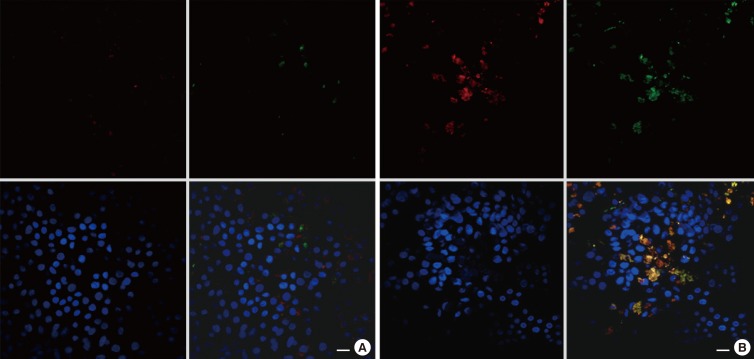
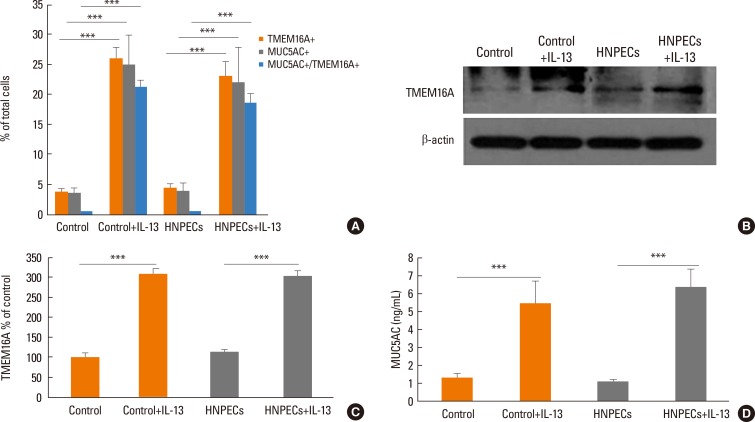
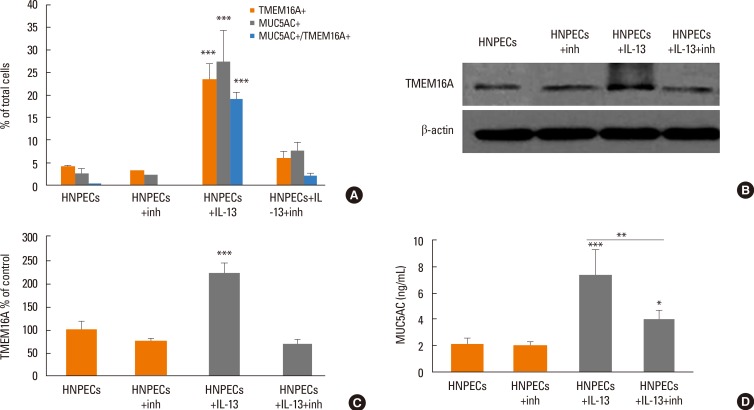
Human sinonasal mucosa tissue and dissociated sinonasal epithelium from control subjects and patients with CRSwNP were assessed for the expression of TMEM16A and the secretion of human mucin 5AC (MUC5AC) by immunohistochemistry, Western blot analysis, and enzyme-linked immuno-sorbent assay (ELISA). A model of the Th2 inflammatory environment was created by exposure of primary air-liquid interface (ALI)-cultured HNPECs to interleukin-13 (IL-13) for 14 days, with subsequent assessment of TMEM16A expression in cell lysates by Western blotting and MUC5AC secretion in apical washings of cells by ELISA.
RESULTS
The expressions of TMEM16A and MUC5AC were increased in human nasal polyp tissue and dissociated nasal polyp epithelium. TMEM16A was detected in IL-13-treated HNPECs, specifically in MUC5AC-positive cells but not in ciliated cells. IL-13 treatment increased percentages of TMEM16A-positive cells, MUC5AC-positive cells, and cells coexpressing TMEM16A/MUC5AC, the expression of TMEM16A protein, and the secretion of MUC5AC. T16Ainh-A01, a TMEM16A inhibitor, attenuated these IL-13-induced effects.
CONCLUSIONS
The expression of TMEM16A and MUC5AC are increased in CRSwNP, which might be a direct effect of Th2 cytokines present in the sinonasal mucosa in CRSwNP. Down-regulation of TMEM16A expression and MUC5AC secretion in HNPECs by T16Ainh-A01 indicates that TMEM16A might play an important role in mucin secretion in upper airway inflammatory diseases.
Keyword
MeSH Terms
Figure
Reference
-
2. Drake-Lee AB, Lowe D, Swanston A, Grace A. Clinical profile and recurrence of nasal polyps. J Laryngol Otol. 1984; 98:783–793. PMID: 6470574.
Article3. Settipane GA, Klein DE, Settipane RJ. Nasal polyps. State of the art. Rhinol Suppl. 1991; 11:33–36. PMID: 1888555.4. Ramanathan M Jr, Lee WK, Spannhake EW, Lane AP. Th2 cytokines associated with chronic rhinosinusitis with polyps down-regulate the antimicrobial immune function of human sinonasal epithelial cells. Am J Rhinol. 2008; 22:115–121. PMID: 18416964.
Article5. Park SJ, Kim TH, Jun YJ, Lee SH, Ryu HY, Jung KJ, et al. Chronic rhinosinusitis with polyps and without polyps is associated with increased expression of suppressors of cytokine signaling 1 and 3. J Allergy Clin Immunol. 2013; 131:772–780. PMID: 23375208.
Article6. Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006; 61:1280–1289. PMID: 17002703.
Article7. Van Bruaene N, Pérez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008; 121:1435–1441. 1441.e1–1441.e3. PMID: 18423831.
Article8. Lu X, Wang N, Long XB, You XJ, Cui YH, Liu Z. The cytokine-driven regulation of secretoglobins in normal human upper airway and their expression, particularly that of uteroglobin-related protein 1, in chronic rhinosinusitis. Respir Res. 2011; 12:28. PMID: 21385388.
Article9. Ocampo C, Suh L, Kern R, Kato A, Conley D, Chandra R, et al. Levels of the cytokines IL-5, IL-13 and Rantes in nasal lavage fluids parallel the cytokine content of nasal polyps in patients with chronic rhinosinusitis with nasal polyps (CRSwNP). J Allergy Clin Immunol. 2013; 131:AB237.
Article10. Nabavi M, Arshi S, Bahrami A, Aryan Z, Bemanian MH, Esmaeilzadeh H, et al. Increased level of interleukin-13, but not interleukin-4 and interferon-γ in chronic rhinosinusitis with nasal polyps. Allergol Immunopathol (Madr). 2014; 42:465–471. PMID: 23969075.
Article11. Bachert C, Zhang N, Holtappels G, De Lobel L, van Cauwenberge P, Liu S, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010; 126:962–968. 968.e1–968.e6. PMID: 20810157.
Article12. Iwashita H, Fujimoto K, Morita S, Nakanishi A, Kubo K. Increased human Ca2+-activated Cl- channel 1 expression and mucus overproduction in airway epithelia of smokers and chronic obstructive pulmonary disease patients. Respir Res. 2012; 13:55. PMID: 22731784.
Article13. Huang F, Wong X, Jan LY. International Union of Basic and Clinical Pharmacology. LXXXV: calcium-activated chloride channels. Pharmacol Rev. 2012; 64:1–15. PMID: 22090471.
Article14. Cunningham SA, Awayda MS, Bubien JK, Ismailov II, Arrate MP, Berdiev BK, et al. Cloning of an epithelial chloride channel from bovine trachea. J Biol Chem. 1995; 270:31016–31026. PMID: 8537359.
Article15. Sun H, Tsunenari T, Yau KW, Nathans J. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc Natl Acad Sci U S A. 2002; 99:4008–4013. PMID: 11904445.
Article16. Qu Z, Wei RW, Mann W, Hartzell HC. Two bestrophins cloned from Xenopus laevis oocytes express Ca2+-activated Cl- currents. J Biol Chem. 2003; 278:49563–49572. PMID: 12939260.
Article17. Suzuki M, Mizuno A. A novel human Cl(-) channel family related to Drosophila flightless locus. J Biol Chem. 2004; 279:22461–22468. PMID: 15010458.
Article18. Galietta LJ. The TMEM16 protein family: a new class of chloride channels? Biophys J. 2009; 97:3047–3053. PMID: 20006941.
Article19. Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008; 134:1019–1029. PMID: 18805094.
Article20. Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008; 455:1210–1215. PMID: 18724360.
Article21. Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008; 322:590–594. PMID: 18772398.
Article22. Tian Y, Kongsuphol P, Hug M, Ousingsawat J, Witzgall R, Schreiber R, et al. Calmodulin-dependent activation of the epithelial calcium-dependent chloride channel TMEM16A. FASEB J. 2011; 25:1058–1068. PMID: 21115851.
Article23. Rock JR, O'Neal WK, Gabriel SE, Randell SH, Harfe BD, Boucher RC, et al. Transmembrane protein 16A (TMEM16A) is a Ca2+-regulated Cl- secretory channel in mouse airways. J Biol Chem. 2009; 284:14875–14880. PMID: 19363029.
Article24. Huang F, Zhang H, Wu M, Yang H, Kudo M, Peters CJ, et al. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc Natl Acad Sci U S A. 2012; 109:16354–16359. PMID: 22988107.
Article25. Namkung W, Phuan PW, Verkman AS. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem. 2011; 286:2365–2374. PMID: 21084298.
Article26. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012; 50:1–12. PMID: 22469599.
Article27. Kondo M, Tamaoki J, Takeyama K, Nakata J, Nagai A. Interleukin-13 induces goblet cell differentiation in primary cell culture from Guinea pig tracheal epithelium. Am J Respir Cell Mol Biol. 2002; 27:536–541. PMID: 12397012.
Article28. Kanoh S, Tanabe T, Rubin BK. IL-13-induced MUC5AC production and goblet cell differentiation is steroid resistant in human airway cells. Clin Exp Allergy. 2011; 41:1747–1756. PMID: 22092504.
Article29. Patou J, Gevaert P, Van Zele T, Holtappels G, van Cauwenberge P, Bachert C. Staphylococcus aureus enterotoxin B, protein A, and lipoteichoic acid stimulations in nasal polyps. J Allergy Clin Immunol. 2008; 121:110–115. PMID: 17980412.
Article30. Scudieri P, Caci E, Bruno S, Ferrera L, Schiavon M, Sondo E, et al. Association of TMEM16A chloride channel overexpression with airway goblet cell metaplasia. J Physiol. 2012; 590:6141–6155. PMID: 22988141.
Article31. Ousingsawat J, Martins JR, Schreiber R, Rock JR, Harfe BD, Kunzelmann K. Loss of TMEM16A causes a defect in epithelial Ca2+-dependent chloride transport. J Biol Chem. 2009; 284:28698–28703. PMID: 19679661.
Article32. Kondo M, Tamaoki J, Takeyama K, Isono K, Kawatani K, Izumo T, et al. Elimination of IL-13 reverses established goblet cell metaplasia into ciliated epithelia in airway epithelial cell culture. Allergol Int. 2006; 55:329–336. PMID: 17075276.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Macrolide on the MUC4 Gene Expression in Human Airway Epithelial Cells
- Effect of Macrolides on the Interleukin-1beta-mediated MUC2/5AC Genes Expression and Mucin Secretion in Human Airway Epithelial Cells
- Interleukin-13 Suppresses MUC5AC Gene Expression and Mucin Secretion in Cultured Normal Human Nasal Epithelial Cells
- Interleukin-1beta-Mediated MUC5AC Gene Expression and Mucin Secretion via PKC-ERK/p38-COX-2-PGE2 in Human Airway Epithelial Cells
- Inhibitory Effect of 15-Deoxy-Delta(12,14)-Prostaglandin J(2) on the MUC8 Expression in the Human Airway Epithelial Cells