Healthc Inform Res.
2010 Mar;16(1):15-21. 10.4258/hir.2010.16.1.15.
An Improved Auto-Generation System to Obtain Reference Intervals for Laboratory Medicine
- Affiliations
-
- 1Department of Laboratory Medicine and Biomedical Informatics, College of Medicine, Pusan National University, Busan, Korea.
- 2Department of Nursing, College of Nursing, Kyungpook National University, Daegu, Korea.
- 3Department of Medical Informatics, College of Medicine, Kyungpook National University, Daegu, Korea. pulala@paran.com
- 4MIPTH, Kyungpook National University, Daegu, Korea.
- KMID: 2166561
- DOI: http://doi.org/10.4258/hir.2010.16.1.15
Abstract
OBJECTIVES
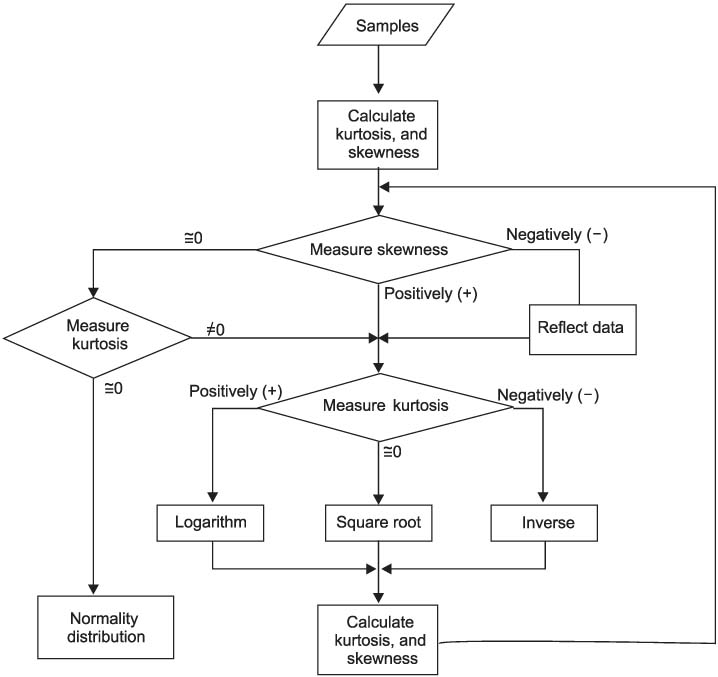
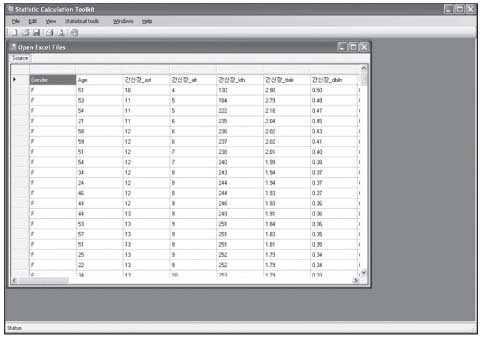
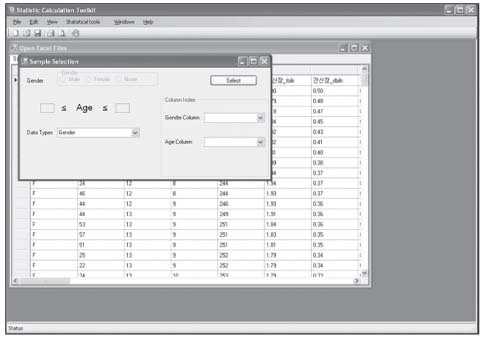
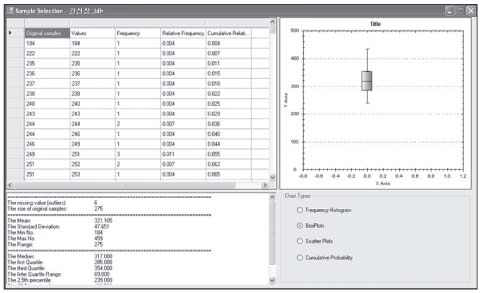
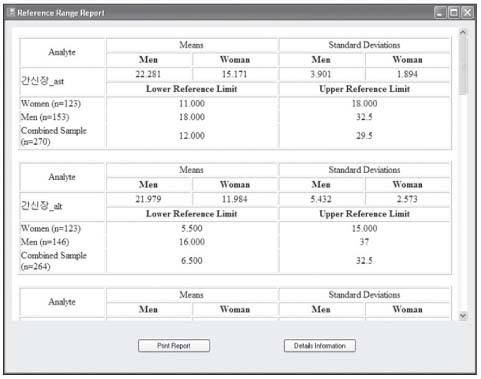
Reference values are highly required parameters for all tests in the clinical laboratory, and the supplementary provision of reliable reference intervals is an important task for both clinical laboratories and diagnostic test manufacturers. Despite the progress that has been made in the conceptual aspects of reference intervals, in practice their use is still not completely satisfactory. Most of the laboratories have used various methods to calculate statistic-based reference intervals, and they have mainly focused on extracted data, yet its use is considerably limited. We had to deal with the inconvenience of using a number of programs (SPSS or SAS, MS Excel) in order to calculate the results of reference intervals. METHODS: In order to obtain standardized reference intervals, we developed an integrated program that can calculate, by a nonparametric method, reference intervals with using the Clinical and Laboratory Standards Institute (CLSI) processes as its guideline. We also developed a grouping interface that enables users to customize classification of each group (age, gender, blood group, race, etc) when calculating reference intervals. RESULTS: To verify the developed program, we compared the reference intervals of the current data on 281 persons for 8 total areas, and the reference intervals were was already calculated beforehand with by using this new program. As a result, both results perfectly matched. CONCLUSIONS: This integrated program will be convenience for calculating reasonable values through continual datainspection at an inspection lab for calculating reference intervals. The newly developed program will improve the consistency and reliability of the statistics on reference intervals.
Keyword
MeSH Terms
Figure
Reference
-
1. Lee JC, Kim SK, Lee CK, Lee SG, Lee HS, Cho KJ. Establishment of reference value using Korean adult medical checkup data and interpretation of test results. J Lab Med Qual Assur. 2006. 28:229–237.2. Sasse EA. Objective evaluation of data in screening for disease. Clin Chim Acta. 2002. 315:17–30.
Article3. Clinical and Laboratory Standards Institute. How to define and determine reference intervals in the clinical laboratory: approved guidelines. 2000. 2nd ed. Wayne (PA): Clinical and Laboratory Standards Institute;1–36.4. PetitClerc C, Wilding P. International Federation of Clinical Chemistry (IFCC), Scientific Committee, Clinical Section. The theory of reference values. Part 2. Selection of individuals for the production of reference values. J Clin Chem Clin Biochem. 1984. 22:203–208.5. Alstrom T, Grasbeck R, Hjelm M, Skandsen S. Recommendations concerning the collection of reference values in clinical chemistry and activity report by the Committee on Reference Values of the Scandinavian Society for Clinical Chemistry and Clinical Physiology. Scand J Clin Lab Invest Suppl. 1975. 144:1–44.6. Berg B, Solberg HE, Nilsson JE, Tryding N. Solberg HE, Grasbeck R, Alstrom T, editors. Practical experience in the selection and preparation of reference individuals: empirical testing of the provisional Scandinavian recommendations. Reference values in laboratory medicine. 1981. Chichester (UK): John Wiley & Sons;55–64.7. Statland BE, Winkel P. Reference values: are they useful? Clin Lab Med. 1984. 4:61–70.
Article8. Reed AH, Henry RJ, Mason WB. Influence of statistical method used on the resulting estimate of normal range. Clin Chem. 1971. 17:275–284.
Article9. Linnet K. Two-stage transformation systems for normalization of reference distributions evaluated. Clin Chem. 1987. 33:381–386.
Article10. Dixon WJ. Processing data for outliers. Biometrics. 1953. 9:74–89.
Article11. Sinton TJ, Cowley DM, Bryant SJ. Reference intervals for calcium, phosphate, and alkaline phosphatase as derived on the basis of multichannel-analyzer profiles. Clin Chem. 1986. 32:76–79.
Article12. Grasbeck R. The evolution of the reference value concept. Clin Chem Lab Med. 2004. 42:692–697.13. PetitClerc C, Wilding P. International Federation of Clinical Chemistry (IFCC), Scientific Committee, Clinical Section. The theory of reference values. Part 2. Selection of individuals for the production of reference values. J Clin Chem Clin Biochem. 1984. 22:203–208.14. Solberg HE, PetitClerc C. Approved recommendation (1988) on the theory of reference values. Part 3. Preparation of individuals and collection of specimens for the production of reference values. Clin Chim Acta. 1988. 177:S3–S11.
Article15. Dybkaer R. International federation of clinical chemistry (IFCC)1),2) the theory of reference values. Part 6. Presentation of observed values related to reference values. J Clin Chem Clin Biochem. 1982. 20:841–845.16. Grossi E, Colombo R, Cavuto S, Franzini C. The REALAB project: a new method for the formulation of reference intervals based on current data. Clin Chem. 2005. 51:1232–1240.
Article17. Henny J, Petitclerc C, Fuentes-Arderiu X, Petersen PH, Queraltó JM, Schiele F, Siest G. Need for revisiting the concept of reference values. Clin Chem Lab Med. 2000. 38:589–595.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Design and Laboratory Implementation of Web Application for Collaboratively Setting Reference Intervals
- Establishment of Reference Intervals for Serum Insulin-Like Growth Factor I in Korean Adult Population
- Establishment of Pediatric Reference Intervals for Routine Laboratory Tests in Korean Population: A Retrospective Multicenter Analysis
- Establishing Reference Intervals for Complete Blood Cell Count in Healthy Korean Elderly Individuals
- Chinese Pediatric Reference Intervals for Serum Cortisol on IMMULITE 2000