Ann Lab Med.
2021 Mar;41(2):155-170. 10.3343/alm.2021.41.2.155.
Establishment of Pediatric Reference Intervals for Routine Laboratory Tests in Korean Population: A Retrospective Multicenter Analysis
- Affiliations
-
- 1Department of Laboratory Medicine, Seoul National University Hospital and College of Medicine, Seoul, Korea
- 2Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea
- 3Department of Laboratory Medicine, Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea
- 4Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 5Department of Biostatistics, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, Korea
- KMID: 2512680
- DOI: http://doi.org/10.3343/alm.2021.41.2.155
Abstract
- Background
Reference intervals defined for adults or children of other ethnicities cannot be applied in the evaluation of Korean pediatric patients. Pediatric reference intervals are difficult to establish because children are in their growing stage and their physiology changes continuously. We aimed to establish reference intervals for routine laboratory tests for Korean pediatric patients through retrospective multicenter data analysis.
Methods
Preoperative laboratory test results from 1,031 pediatric patients aged 0 month–18 years who underwent minor surgeries in four university hospitals were collected. Age- and sex-specific reference intervals for routine laboratory tests were defined based on the Clinical and Laboratory Standards Institute (CLSI) EP28-A3c guidelines.
Results
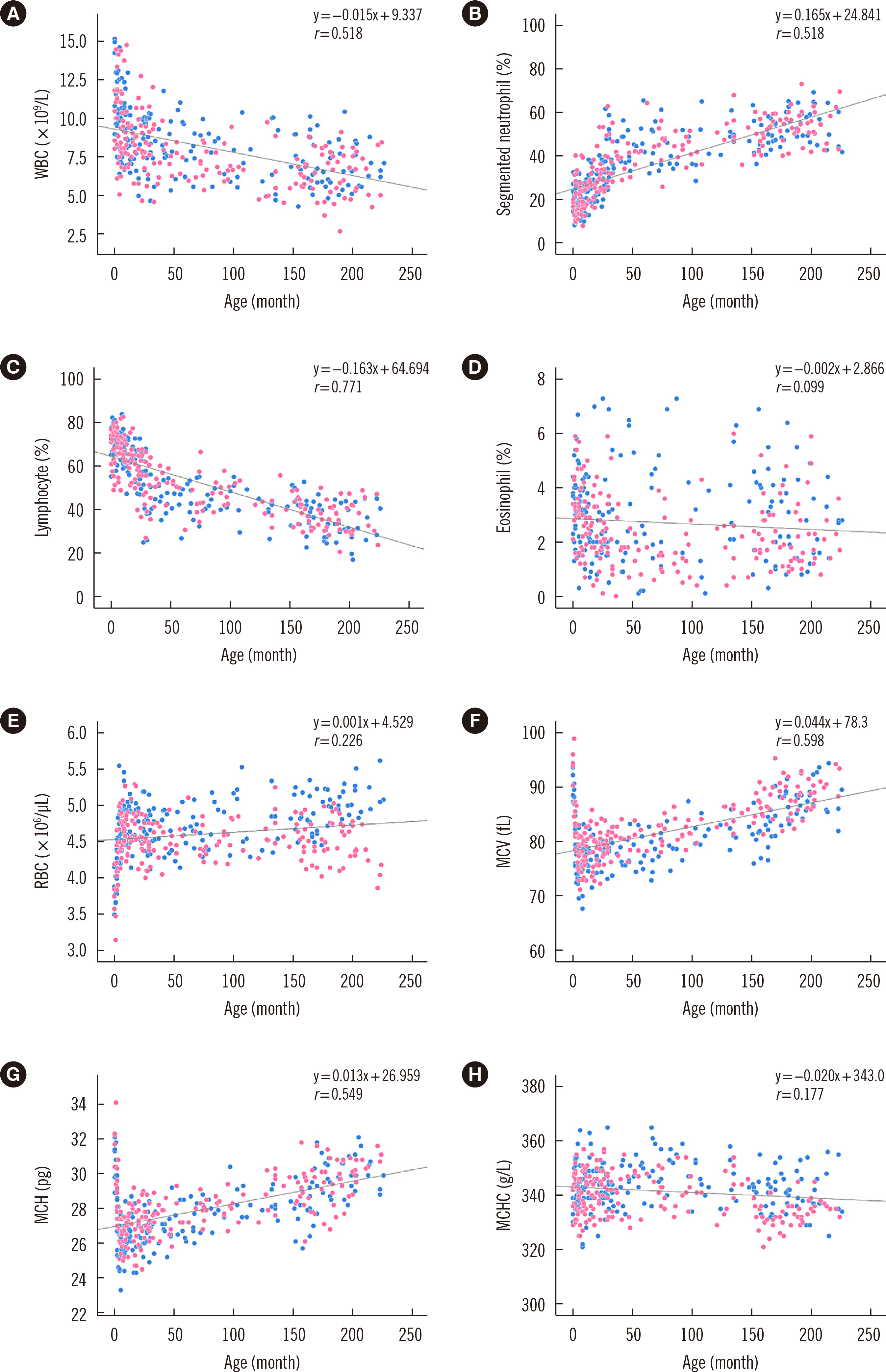
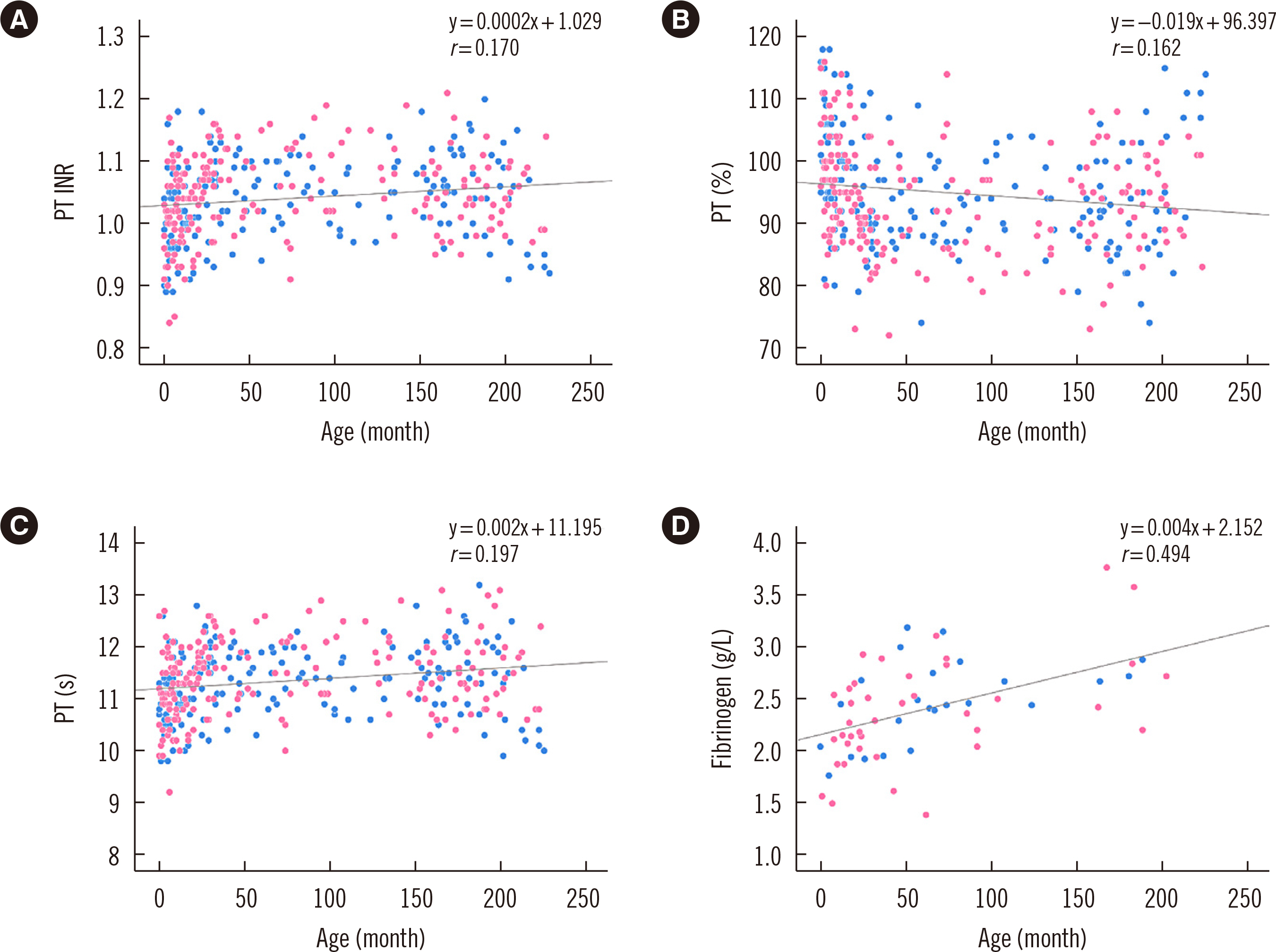
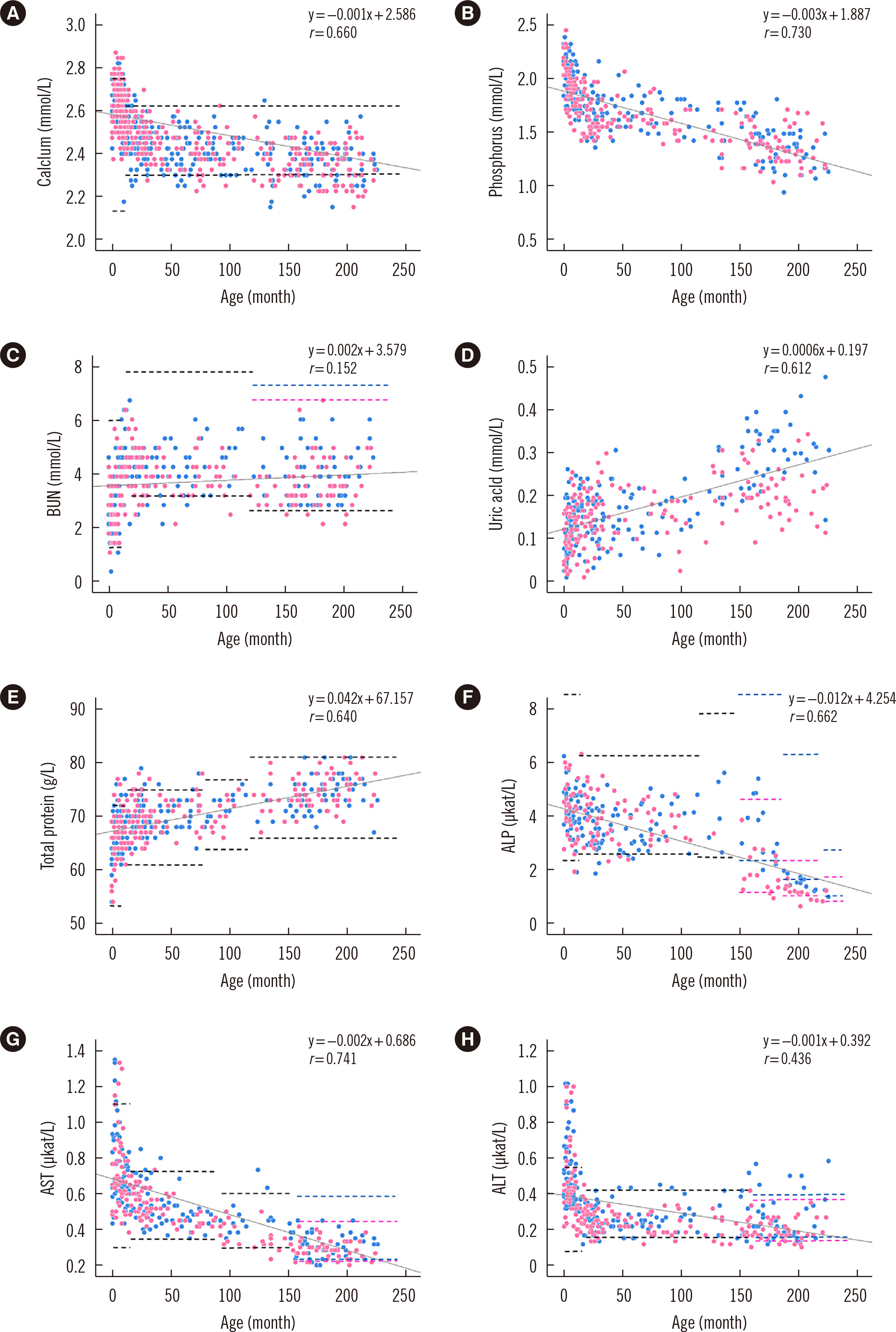
The pediatric reference intervals determined in this study were different from existing adult reference intervals and pediatric reference intervals for other ethnicities. Most tests required age-specific partitioning, and some of those required sex-specific partitioning for at least one age-partitioned subgroup. Erythrocyte sedimentation rate, monocyte percentage, basophil percentage, activated partial thromboplastin time, glucose, cholesterol, albumin, bilirubin, chloride, and C-reactive protein did not show any difference between age- or sex-partitioned subgroups.
Conclusions
We determined Korean pediatric reference intervals for hematology, coagulation, and chemistry tests by indirect sampling based on medical record data from multiple institutions. These reference intervals would be valuable for clinical evaluations in the Korean pediatric population.
Keyword
Figure
Cited by 3 articles
-
Age-specific Reference Intervals for Anti-Müllerian Hormone
Haeil Park
Ann Lab Med. 2022;42(6):617-618. doi: 10.3343/alm.2022.42.6.617.Indirect Method for Estimation of Reference Intervals of Inflammatory Markers
Taewon Kang, Jeaeun Yoo, Dong Wook Jekarl, Hyojin Chae, Myungshin Kim, Yeon-Joon Park, Eun-Jee Oh, Yonggoo Kim
Ann Lab Med. 2023;43(1):55-63. doi: 10.3343/alm.2023.43.1.55.Functional Reference Limits: Describing Physiological Relationships and Determination of Physiological Limits for Enhanced Interpretation of Laboratory Results
Tyng Yu Chuah, Chun Yee Lim, Rui Zhen Tan, Busadee Pratumvinit, Tze Ping Loh, Samuel Vasikaran, Corey Markus
Ann Lab Med. 2023;43(5):408-417. doi: 10.3343/alm.2023.43.5.408.
Reference
-
1. Horn PS, Pesce AJ. 2003; Reference intervals: an update. Clin Chim Acta. 334:5–23. DOI: 10.1016/S0009-8981(03)00133-5. PMID: 12867273.
Article2. CLSI. 2010. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline. CLSI EP28-A3c. Third ed. Clinical Laboratory Standards Institute;Wayne, PA:3. Jung B, Adeli K. 2009; Clinical laboratory reference intervals in pediatrics: the CALIPER initiative. Clin Biochem. 42:1589–95. DOI: 10.1016/j.clinbiochem.2009.06.025. PMID: 19591815.
Article4. Schnabl K, Chan MK, Adeli K. 2008; 3. Pediatric reference intervals: critical gap analysis and establishment of a national initiative. EJIFCC. 19:115–22. PMID: 27683306. PMCID: PMC4975206.5. Schnabl K, Chan MK, Gong Y, Adeli K. 2008; Closing the gaps in paediatric reference intervals: the CALIPER initiative. Clin Biochem Rev. 29:89–96. PMID: 19107221. PMCID: PMC2605413.6. Adeli K. 2011; Closing the gaps in pediatric reference intervals: the CALIPER initiative. Clin Biochem. 44:480–2. DOI: 10.1016/j.clinbiochem.2011.02.017. PMID: 22036336.
Article7. Chan MK, Seiden-Long I, Aytekin M, Quinn F, Ravalico T, Ambruster D, et al. 2009; Canadian Laboratory Initiative on Pediatric Reference Interval Database (CALIPER): pediatric reference intervals for an integrated clinical chemistry and immunoassay analyzer, Abbott ARCHITECT ci8200. Clin Biochem. 42:885–91. DOI: 10.1016/j.clinbiochem.2009.01.014. PMID: 19318027.
Article8. Colantonio DA, Kyriakopoulou L, Chan MK, Daly CH, Brinc D, Venner AA, et al. 2012; Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem. 58:854–68. DOI: 10.1373/clinchem.2011.177741. PMID: 22371482.
Article9. Kohse KP, Thamm M. 2011; KiGGS the German survey on children's health as data base for reference intervals. Clin Biochem. 44:479. DOI: 10.1016/j.clinbiochem.2011.02.016. PMID: 22036335.10. Kurth BM, Kamtsiuris P, Hölling H, Schlaud M, Dölle R, Ellert U, et al. 2008; The challenge of comprehensively mapping children's health in a nation-wide health survey: design of the German KiGGS-Study. BMC Public Health. 8:196. DOI: 10.1186/1471-2458-8-196. PMID: 18533019.
Article11. Flanders MM, Crist RA, Roberts WL, Rodgers GM. 2005; Pediatric reference intervals for seven common coagulation assays. Clin Chem. 51:1738–42. DOI: 10.1373/clinchem.2005.050211. PMID: 16120957.
Article12. Clifford SM, Bunker AM, Jacobsen JR, Roberts WL. 2011; Age and gender specific pediatric reference intervals for aldolase, amylase, ceruloplasmin, creatine kinase, pancreatic amylase, prealbumin, and uric acid. Clin Chim Acta. 412:788–90. DOI: 10.1016/j.cca.2011.01.011. PMID: 21238443.
Article13. Bailey D, Colantonio D, Kyriakopoulou L, Cohen AH, Chan MK, Armbruster D, et al. 2013; Marked biological variance in endocrine and biochemical markers in childhood: establishment of pediatric reference intervals using healthy community children from the CALIPER cohort. Clin Chem. 59:1393–405. DOI: 10.1373/clinchem.2013.204222. PMID: 23637247.
Article14. Tahmasebi H, Asgari S, Hall A, Higgins V, Chowdhury A, Thompson R, et al. 2019; Influence of ethnicity on biochemical markers of health and disease in the CALIPER cohort of healthy children and adolescents. Clin Chem Lab Med. 58:605–17. DOI: 10.1515/cclm-2019-0876. PMID: 31874092.
Article15. Cho SM, Lee SG, Kim HS, Kim JH. 2014; Establishing pediatric reference intervals for 13 biochemical analytes derived from normal subjects in a pediatric endocrinology clinic in Korea. Clin Biochem. 47:268–71. DOI: 10.1016/j.clinbiochem.2014.09.010. PMID: 25241678.
Article16. Nah EH, Kim S, Cho S, Cho HI, Nah EH. 2018; Complete Blood Count Reference Intervals and Patterns of Changes Across Pediatric, Adult, and Geriatric Ages in Korea. Ann Lab Med. 38:503–511. DOI: 10.3343/alm.2018.38.6.503. PMID: 30027692. PMCID: PMC6056383.
Article17. Lee HR, Shin S, Yoon JH, Roh EY, Chang JY. 2016; Reference Intervals of Hematology and Clinical Chemistry Analytes for 1-Year-Old Korean Children. Ann Lab Med. 36:481–8. DOI: 10.3343/alm.2016.36.5.481. PMID: 27374715. PMCID: PMC4940493.
Article18. Kim B, Lee MN, Park HD, Kim JW, Chang YS, Park WS, Lee SY. 2015; Dried blood spot testing for seven steroids using liquid chromatography-tandem mass spectrometry with reference interval determination in the Korean population. Ann Lab Med. 35:578–85. DOI: 10.3343/alm.2015.35.6.578. PMID: 26354345. PMCID: PMC4579101.
Article19. Ceriotti F. 2012; Establishing pediatric reference intervals: a challenging task. Clin Chem. 58:808–10. DOI: 10.1373/clinchem.2012.183483. PMID: 22377530.
Article20. Shaw JL, Binesh Marvasti T, Colantonio D, Adeli K. 2013; Pediatric reference intervals: challenges and recent initiatives. Crit Rev Clin Lab Sci. 50:37–50. DOI: 10.3109/10408363.2013.786673. PMID: 23656169.
Article21. Farrell CL, Nguyen L. 2019; Indirect reference intervals: harnessing the power of stored laboratory data. Clin Biochem Rev. 40:99–111. DOI: 10.33176/AACB-19-00022. PMID: 31205377. PMCID: PMC6544248.22. Beck GR, Sullivan EC, Moran E, Zerler B. 1998; Relationship between alkaline phosphatase levels, osteopontin expression, and mineralization in differentiating MC3T3‐E1 osteoblasts. J Cell Biochem. 68:269–80. DOI: 10.1002/(SICI)1097-4644(19980201)68:2<269::AID-JCB13>3.0.CO;2-A.
Article23. Stein GS, Lian JB, Owen TA. 1990; Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 4:3111–23. DOI: 10.1096/fasebj.4.13.2210157. PMID: 2210157.
Article24. Clifford SM, Bunker AM, Jacobsen JR, Roberts WL. 2011; Age and gender specific pediatric reference intervals for aldolase, amylase, ceruloplasmin, creatine kinase, pancreatic amylase, prealbumin, and uric acid. Clin Chim Acta. 412:788–90. DOI: 10.1016/j.cca.2011.01.011. PMID: 21238443.
Article25. Bureau International des Poids et Mesures. JCTLM database: laboratory medicine and in vitro diagnostics. https://www.bipm.org/jctlm/. Updated on Jan 2020.26. Solberg HE, Lahti A. 2005; Detection of outliers in reference distributions: performance of Horn's algorithm. Clin Chem. 51:2326–32. DOI: 10.1373/clinchem.2005.058339. PMID: 16223885.
Article27. Horn PS, Pesce AJ, Copeland BE. 1998; A robust approach to reference interval estimation and evaluation. Clin Chem. 44:622–31. DOI: 10.1093/clinchem/44.3.622. PMID: 9510871.
Article28. Shaw JL, Cohen A, Konforte D, Binesh-Marvasti T, Colantonio DA, Adeli K. 2014; Validity of establishing pediatric reference intervals based on hospital patient data: a comparison of the modified Hoffmann approach to CALIPER reference intervals obtained in healthy children. Clin Biochem. 47:166–72. DOI: 10.1016/j.clinbiochem.2013.11.008. PMID: 24316101.
Article29. Solberg HE. 1994; Using a hospitalized population to establish reference intervals: pros and cons. Clin Chem. 40:2205–6. DOI: 10.1093/clinchem/40.12.2205. PMID: 7988005.
Article30. Arzideh F, Brandhorst G, Gurr E, Hinsch W, Hoff T, Roggenbuck L, et al. 2009; An improved indirect approach for determining reference limits from intra-laboratory data bases exemplified by concentrations of electrolytes. J Lab Med. 33:52–66. DOI: 10.1515/JLM.2009.015.31. Hoffmann RG. 1963; Statistics in the practice of medicine. JAMA. 185:864–73. DOI: 10.1001/jama.1963.03060110068020. PMID: 14043090.
Article32. Zierk J, Arzideh F, Rechenauer T, Haeckel R, Rascher W, Metzler M, et al. 2015; Ageand sex-specific dynamics in 22 hematologic and biochemical analytes from birth to adolescence. Clin Chem. 61:964–73. DOI: 10.1373/clinchem.2015.239731. PMID: 25967371.33. Jones GRD, Haeckel R, Loh TP, Sikaris K, Streichert T, Katayev A, et al. 2018; Indirect methods for reference interval determination-review and recommendations. Clin Chem Lab Med. 57:20–9. DOI: 10.1515/cclm-2018-0073. PMID: 29672266.34. Estey MP, Cohen AH, Colantonio DA, Chan MK, Marvasti TB, Randell E, et al. 2013; CLSI-based transference of the CALIPER database of pediatric reference intervals from Abbott to Beckman, Ortho, Roche and Siemens Clinical Chemistry Assays: direct validation using reference samples from the CALIPER cohort. Clin Biochem. 46:1197–219. DOI: 10.1016/j.clinbiochem.2013.04.001. PMID: 23578738.
Article35. Araújo PA, Thomas D, Sadeghieh T, Bevilacqua V, Chan MK, Chen Y, et al. 2015; CLSI-based transference of the CALIPER database of pediatric reference intervals to Beckman Coulter DxC biochemical assays. Clin Biochem. 48:870–80. DOI: 10.1016/j.clinbiochem.2015.06.002. PMID: 26070714.
Article36. Abou El Hassan M, Stoianov A, Araújo PA, Sadeghieh T, Chan MK, Chen Y, et al. 2015; CLSI-based transference of CALIPER pediatric reference intervals to Beckman Coulter AU biochemical assays. Clin Biochem. 48:1151–9. DOI: 10.1016/j.clinbiochem.2015.05.002. PMID: 25979809.
Article37. CLSI. 2013. Measurement procedure comparison and bias estimation using patient samples; approved guideline. CLSI EP09-A3. Third ed. Clinical Laboratory Standards Institute;Wayne, PA:
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Establishment of Trimester-Specific Reference Intervals for Thyroid Hormones in Korean Pregnant Women
- An Improved Auto-Generation System to Obtain Reference Intervals for Laboratory Medicine
- Establishment of Reference Intervals of Tumor Markers in Korean Adults
- Influence of Waist Circumference Index of Metabolic Syndrome on the Establishment of Reference Intervals
- Clinical chemistry values in elderly Korean people: single institutional study