Clin Exp Otorhinolaryngol.
2016 Mar;9(1):56-61. 10.21053/ceo.2016.9.1.56.
Histological Effect of Basic Fibroblast Growth Factor on Chronic Vocal Fold Scarring in a Rat Model
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Graduate School of Medicine, Kyoto University, Kyoto, Japan. tateya@ent.kuhp.kyoto-u.ac.jp
- 2Institute for Virus Research, Kyoto University, Kyoto, Japan.
- 3The Hakubi Center, Kyoto University, Kyoto, Japan.
- 4Department of Otorhinolaryngology-Head and Neck Surgery, Kyungpook National University School of Medicine, Daegu, Korea.
- 5Division of Otolaryngology-Head and Neck Surgery, University of Wisconsin-Madison, Madison, WI, USA.
- KMID: 2166290
- DOI: http://doi.org/10.21053/ceo.2016.9.1.56
Abstract
OBJECTIVES
Vocal fold scarring is one of the most challenging laryngeal disorders to treat and there are currently no consistently effective treatments available. Our previous studies have shown the therapeutic potential of basic fibroblast growth factor (bFGF) for vocal fold scarring. However, the histological effects of bFGF on scarred vocal fold have not been elucidated. The aim of this study was to examine the histological effects of bFGF on chronic vocal fold scarring.
METHODS
Sprague-Dawley rats were divided into phosphate buffered saline (sham) and bFGF groups. Unilateral vocal fold stripping was performed and the drug was injected into the scarred vocal fold for each group 2 months postoperatively. Injections were performed weekly for 4 weeks. Two months after the last injection, larynges were harvested and histologically analyzed.
RESULTS
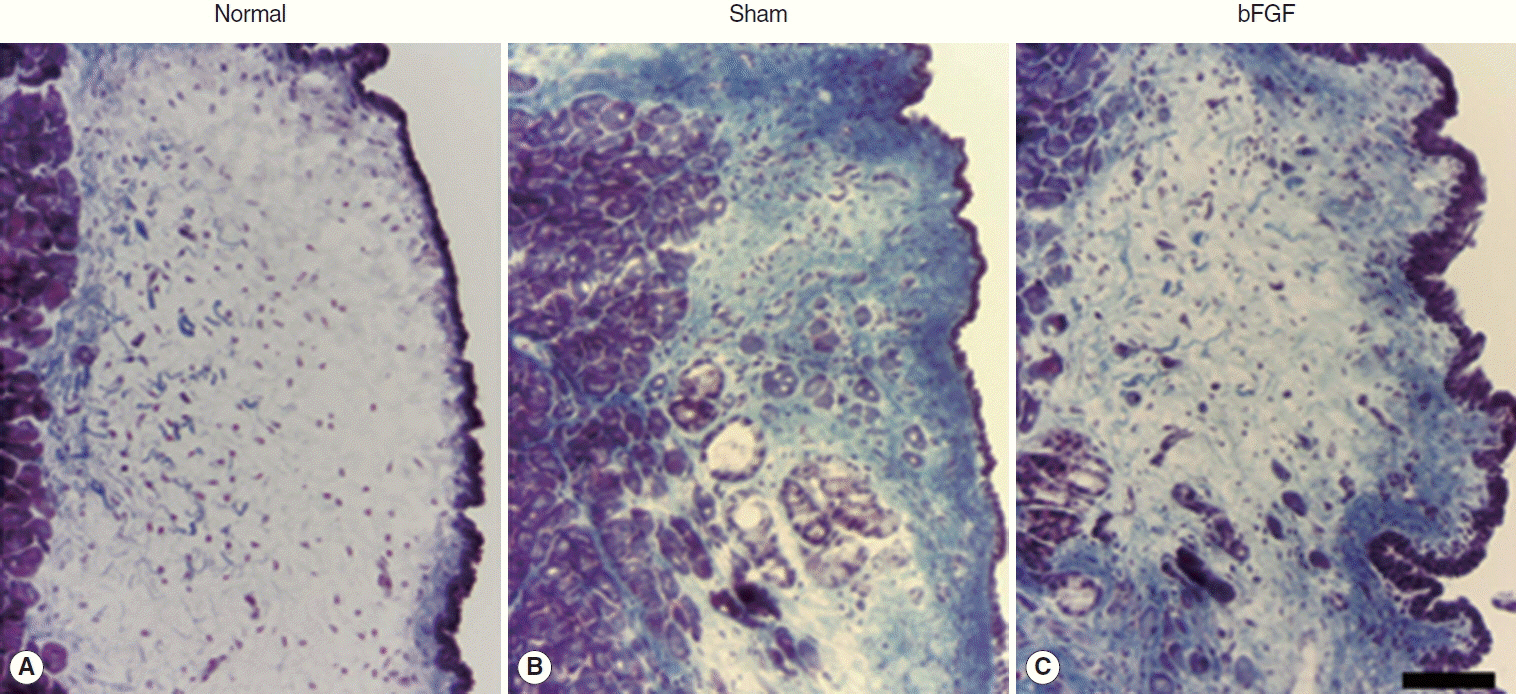
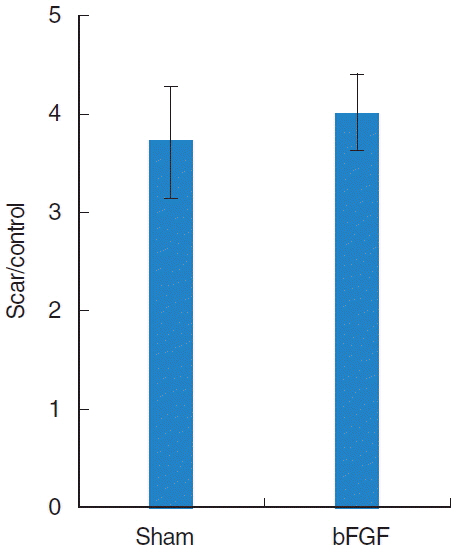
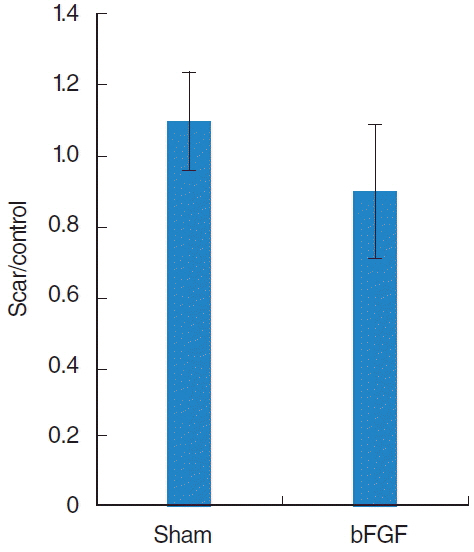
A significant increase of hyaluronic acid was observed in the vocal fold of the bFGF group compared with that of the sham group. However, there was no remarkable change in collagen expression nor in vocal fold contraction.
CONCLUSION
Significant increase of hyaluronic acid by local bFGF injection was thought to contribute to the therapeutic effects on chronic vocal fold scarring.
MeSH Terms
Figure
Reference
-
1. Gray SD, Titze IR, Alipour F, Hammond TH. Biomechanical and histologic observations of vocal fold fibrous proteins. Ann Otol Rhinol Laryngol. 2000; Jan. 109(1):77–85.
Article2. Thibeault SL, Gray SD, Bless DM, Chan RW, Ford CN. Histologic and rheologic characterization of vocal fold scarring. J Voice. 2002; Mar. 16(1):96–104.
Article3. Chan RW, Gray SD, Titze IR. The importance of hyaluronic acid in vocal fold biomechanics. Otolaryngol Head Neck Surg. 2001; Jun. 124(6):607–14.
Article4. Benninger MS, Alessi D, Archer S, Bastian R, Ford C, Koufman J, et al. Vocal fold scarring: current concepts and management. Otolaryngol Head Neck Surg. 1996; Nov. 115(5):474–82.
Article5. Woo P, Casper J, Colton R, Brewer D. Diagnosis and treatment of persistent dysphonia after laryngeal surgery: a retrospective analysis of 62 patients. Laryngoscope. 1994; Sep. 104(9):1084–91.6. Rousseau B, Hirano S, Scheidt TD, Welham NV, Thibeault SL, Chan RW, et al. Characterization of vocal fold scarring in a canine model. Laryngoscope. 2003; Apr. 113(4):620–7.
Article7. Tateya T, Tateya I, Sohn JH, Bless DM. Histologic characterization of rat vocal fold scarring. Ann Otol Rhinol Laryngol. 2005; Mar. 114(3):183–91.
Article8. Tateya T, Tateya I, Sohn JH, Bless DM. Histological study of acute vocal fold injury in a rat model. Ann Otol Rhinol Laryngol. 2006; Apr. 115(4):285–92.
Article9. Ford CN, Bless DM, Campbell D. Studies of injectable soluble collagen for vocal fold augmentation. Rev Laryngol Otol Rhinol (Bord). 1987; 108(1):33–6.10. Ford CN, Staskowski PA, Bless DM. Autologous collagen vocal fold injection: a preliminary clinical study. Laryngoscope. 1995; Sep. 105(9 Pt 1):944–8.
Article11. Kriesel KJ, Thiebault SL, Chan RW, Suzuki T, VanGroll PJ, Bless DM, et al. Treatment of vocal fold scarring: rheological and histological measures of homologous collagen matrix. Ann Otol Rhinol Laryngol. 2002; Oct. 111(10):884–9.
Article12. Wexler DB, Jiang J, Gray SD, Titze IR. Phonosurgical studies: fat-graft reconstruction of injured canine vocal cords. Ann Otol Rhinol Laryngol. 1989; Sep. 98(9):668–73.
Article13. Hirano S, Bless DM, Nagai H, Rousseau B, Welham NV, Montequin DW, et al. Growth factor therapy for vocal fold scarring in a canine model. Ann Otol Rhinol Laryngol. 2004; Oct. 113(10):777–85.
Article14. Hong HH, Trackman PC. Cytokine regulation of gingival fibroblast lysyl oxidase, collagen, and elastin. J Periodontol. 2002; Feb. 73(2):145–52.
Article15. Palmon A, Roos H, Reichenberg E, Grosskop A, Bar Kana I, Pitaru S, et al. Basic fibroblast growth factor suppresses tropoelastin gene expression in cultured human periodontal fibroblasts. J Periodontal Res. 2001; Apr. 36(2):65–70.
Article16. Suehiro A, Hirano S, Kishimoto Y, Tateya I, Rousseau B, Ito J. Effects of basic fibroblast growth factor on rat vocal fold fibroblasts. Ann Otol Rhinol Laryngol. 2010; Oct. 119(10):690–6.
Article17. Welham NV, Montequin DW, Tateya I, Tateya T, Choi SH, Bless DM. A rat excised larynx model of vocal fold scar. J Speech Lang Hear Res. 2009; Aug. 52(4):1008–20.
Article18. Hirano S, Mizuta M, Kaneko M, Tateya I, Kanemaru S, Ito J. Regenerative phonosurgical treatments for vocal fold scar and sulcus with basic fibroblast growth factor. Laryngoscope. 2013; Nov. 123(11):2749–55.
Article19. Inagi K, Connor NP, Ford CN, Schultz E, Rodriquez AA, Bless DM, et al. Physiologic assessment of botulinum toxin effects in the rat larynx. Laryngoscope. 1998; Jul. 108(7):1048–54.
Article20. Suzuki T, Connor NP, Lee K, Leverson G, Ford CN. Laryngeal-respiratory kinematics are impaired in aged rats. Ann Otol Rhinol Laryngol. 2002; Aug. 111(8):684–9.
Article21. Hirano S, Bless DM, Rousseau B, Welham N, Montequin D, Chan RW, et al. Prevention of vocal fold scarring by topical injection of hepatocyte growth factor in a rabbit model. Laryngoscope. 2004; Mar. 114(3):548–56.
Article22. Heldin P, Laurent TC, Heldin CH. Effect of growth factors on hyaluronan synthesis in cultured human fibroblasts. Biochem J. 1989; Mar. 258(3):919–22.
Article23. Hirano S, Bless DM, del Río AM, Connor NP, Ford CN. Therapeutic potential of growth factors for aging voice. Laryngoscope. 2004; Dec. 114(12):2161–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Surgical Treatment of Intractable Vocal Fold Scar Using Basic Fibroblast Growth Factor and Collagen Scaffold
- The Efficacy of Fibroblast Growth Factor for the Treatment of Chronic Vocal Fold Scarring: From Animal Model to Clinical Application
- Effect of Basic Fibroblast Growth Factor on the Regeneration of the Allografted Sciatic Nerve in Rat
- Effects of Neurotrophic Factor on Fetal Mesencephalic Grafts in Parkinsonian Rat Models
- Vocal Fold Injection: Review of Indications, Techniques, and Materials for Augmentation