Cancer Res Treat.
2008 Mar;40(1):22-26.
Gemcitabine versus Gemcitabine Combined with Cisplatin Treatment Locally Advanced or Metastatic Pancreatic Cancer: A Retrospective Analysis
- Affiliations
-
- 1Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea. kimhj@dau.ac.kr
- 2Department of Surgery, Dong-A University College of Medicine, Busan, Korea.
- 3Department of Pathology, Dong-A University College of Medicine, Busan, Korea.
Abstract
-
PURPOSE: Gemcitabine is the most active agent to treat unresectable pancreatic cancer. The superiority of combining other drugs with cisplatin is still controversial; therefore, we performed a retrospective analysis of gemcitabine versus gemcitabine combined with cisplatin to determine the treatment outcomes for patients with locally advanced or metastatic pancreatic cancer.
MATERIALS AND METHODS
From 2001 to 2007, we enrolled 60 patients who were treated with gemcitabine or gemcitabine combined with cisplatin for locally advanced or metastatic pancreatic cancer. Gemcitabine 1, 000 mg/m2 (G) was administrated at day 1 and day 8 every 3 weeks. Cisplatin 60 mg/m2 was added at day 1 every 3 weeks to the gemcitabine schedule (GP).
RESULTS
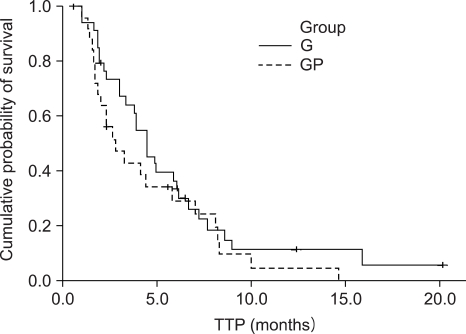
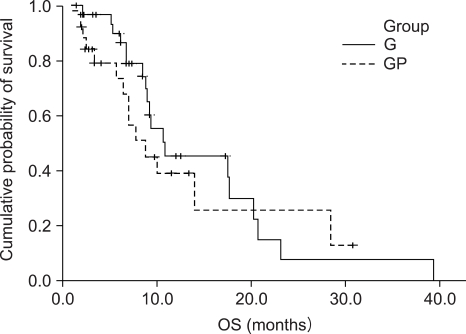
Number of G: GP was 34: 26, locally advanced to metastatic ratio was 35% to 65% in group G and 46% to 54% in group GP. Median follow up duration was 29 months. The median number of chemotherapy cycles was 4 (range: 2~11) for the G group, and 4 (range: 1~11) for the GP group. The response rate of the G and GP groups was 17% and 11%, respectively. The progression free survival (PFS) was 4.5 months and 2.8 months, respectively, for the G and GP groups. The overall survival (OS) was 10.7 and 8.7 months respectively, for the G and GP groups, but there is no statistically significant difference of the PFS (p=0.2396) and OS (p=0.4643) between the 2 groups. The hematological toxicity profile was similar (the grade III neutropenia and thrombocytopenia was 4.4% and 3.1%, respectively, in G group, and 7.5% and 2.8%, respectively, in the GP group). But non-hematological toxicities such as skin rash, abnormal liver function and nausea/vomiting were observed in 3 patients of the GP group. On the prognostic factor analysis, no factors predicted a longer PFS and OS for both the G and GP groups.
CONCLUSIONS
Gemcitabine single treatment might be more tolerable and it had the same efficacy compared to cisplatin combination treatment in this retrospective study.
Keyword
MeSH Terms
Figure
Reference
-
1. Shin HR, Jung KW, Won YJ, Park JG. 2002 Annual report of the Korea central cancer registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004; 36:103–114.
Article2. Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997; 15:2403–2413. PMID: 9196156.
Article3. Palmer KR, Kerr M, Knowles G, Cull A, Carter DC, Leonard RC. Chemotherapy prolongs survival in inoperable pancreatic carcinoma. Br J Surg. 1994; 81:882–885. PMID: 8044610.
Article4. Glimelius B, Hoffman K, Sjoden PO, Jacobsson G, Sellstrom H, Enander LK, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996; 7:593–600. PMID: 8879373.
Article5. Rothenberg ML, Moore MJ, Cripps MC, Andersen JS, Portenoy RK, Burris HA 3rd, et al. A phase II trial of gemcitabine in patients with 5-FU-refractory pancreas cancer. Ann Oncol. 1996; 7:347–353. PMID: 8805925.
Article6. Wils JA, Kok T, Wagener DJ, Selleslags J, Duez N. Activity of cisplatin in adenocarcinoma of the pancreas. Eur J Cancer. 1993; 29A:203–204. PMID: 8422283.
Article7. Kanzawa F, Saijo N. In vitro interaction between gemcitabine and other anticancer drugs using a novel three-dimensional model. Semin Oncol. 1997; 24(2):Suppl 7. S7–S8. S7–S16. PMID: 9194474.8. Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Peters GJ. Synergistic interaction between cisplatin and gemcitabine in vitro. Clin Cancer Res. 1996; 2:521–530. PMID: 9816199.9. Colucci G, Giuliani F, Gebbia V, Biglietto M, Rabitti P, Uomo G, et al. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell'Italia Meridionale. Cancer. 2002; 94:902–910. PMID: 11920457.10. Xie DR, Liang HL, Wang Y, Guo SS, Yang Q. Meta-analysis on inoperable pancreatic cancer: a comparison between gemcitabine-based combination therapy and gemcitabine alone. World J Gastroenterol. 2006; 12:6973–6981. PMID: 17109519.
Article11. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–216. PMID: 10655437.12. Storniolo AM, Enas NH, Brown CA, Voi M, Rothenberg ML, Schilsky R. An investigational new drug treatment program for patients with gemcitabine: results for over 3000 patients with pancreatic carcinoma. Cancer. 1999; 85:1261–1268. PMID: 10189130.13. Gansauge F, Ramadani M, Pressmar J, Gansauge S, Muehling B, Stecker K, et al. NSC-631570 (Ukrain) in the palliative treatment of pancreatic cancer. Results of a phase II trial. Langenbecks Arch Surg. 2002; 386:570–574. PMID: 11914932.14. Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007; 25:1960–1966. PMID: 17452677.
Article15. Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schuller J, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007; 25:2212–2217. PMID: 17538165.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy for Pancreatic Cancer
- Long Term Complete Response of Unresectable Locally Advanced Pancreatic Cancer after CCRT and Gemcitabine Chemotherapy
- A Phase II Study of Combination Chemotherapy with Gemcitabine, 5-fluorouracil, and Cisplatin for Advanced Pancreatic Cancer
- Novel Palliative Chemotherapy for Cholangiocarcinoma
- Gemcitabine Therapy in Patients with Advanced Pancreatic Cancer