Cancer Res Treat.
2010 Dec;42(4):185-190.
Oxaliplatin-Induced Chronic Peripheral Neurotoxicity: A Prospective Analysis in Patients with Colorectal Cancer
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. hematoma@skku.edu
- 2Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
Abstract
- PURPOSE
Oxaliplatin-induced chronic peripheral neurotoxicity (OXCPN) manifests as a loss of sensation and dysesthesia in the distal extremities, which may impair daily activities and increase in incidence with the amount of oxaliplatin delivered. The variation in the reported incidence and severity of OXCPN may be a consequence of differences in the baseline characteristics of patients.
MATERIALS AND METHODS
This was a prospective study (ClinicalTrials.gov, NCT00977717) in which OXCPN was recorded for all consecutive colon cancer patients treated at Samsung Medical Center (Seoul, Korea) with oxaliplatin-based combination chemotherapy. The primary endpoint was the incidence of severe OXCPN (grade 2 lasting for >7 days, or grade 3). The association of severe OXCPN and pretreatment parameters was evaluated using a multivariate regression model.
RESULTS
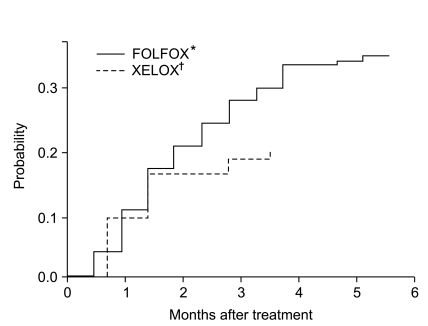
Between Jan 2008 and Feb 2010, 100 patients treated with adjuvant folinic acid/fluorouracil plus oxaliplatin (FOLFOX) and 266 patients treated with capecitabine plus oxaliplatin (XELOX) or FOLFOX for advanced disease were registered into our study. The median cumulative dose of oxaliplatin was 796 mg/m2 (range, 85 to 1,583 mg/m2). Severe OXCPN was observed in 126 (34%) patients. Overall, 43 patients discontinued chemotherapy due to toxicity: 23 without severe OXCPN and 20 with severe OXCPN. In univariate analysis, severe OXCPN was frequently observed in patients with age > or =55 years (p<0.01), stage II or III (p<0.01), adjuvant setting (p=0.01), FOLFOX (p<0.01), performance status of 0 (p=0.02), and those with no prior chemotherapy (p<0.01). In a multivariate regression model, the number of chemotherapy cycles and the cumulative oxaliplatin dose were not associated with the development of severe OXCPN.
CONCLUSION
We failed to find a significant association between patient characteristics at baseline and the development of severe OXCPN after oxaliplatin-based combination chemotherapy. Pharmacogenomic profiling using genome-wide association study in these patients is underway.
Keyword
MeSH Terms
Figure
Reference
-
1. Kelly H, Goldberg RM. Systemic therapy for metastatic colorectal cancer: current options, current evidence. J Clin Oncol. 2005; 23:4553–4560. PMID: 16002847.
Article2. Woynarowski JM, Chapman WG, Napier C, Herzig MC, Juniewicz P. Sequence- and region-specificity of oxaliplatin adducts in naked and cellular DNA. Mol Pharmacol. 1998; 54:770–777. PMID: 9804612.
Article3. Raymond E, Chaney SG, Taamma A, Cvitkovic E. Oxaliplatin: a review of preclinical and clinical studies. Ann Oncol. 1998; 9:1053–1071. PMID: 9834817.
Article4. de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000; 18:2938–2947. PMID: 10944126.
Article5. Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004; 22:23–30. PMID: 14665611.
Article6. Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004; 350:2343–2351. PMID: 15175436.
Article7. Kweekel DM, Gelderblom H, Guchelaar HJ. Pharmacology of oxaliplatin and the use of pharmacogenomics to individualize therapy. Cancer Treat Rev. 2005; 31:90–105. PMID: 15847979.
Article8. Argyriou AA, Polychronopoulos P, Iconomou G, Chroni E, Kalofonos HP. A review on oxaliplatin-induced peripheral nerve damage. Cancer Treat Rev. 2008; 34:368–377. PMID: 18281158.
Article9. Braun AH, Dirsch O, Hilger RA, Schleucher N, Tewes M, Rustum YM, et al. Preclinical evaluation of the combination of epidermal growth factor inhibitor ZD1839 (Iressa) and irinotecan (SN-38) in human colon cancer cells. Proc Am Soc Clin Oncol. 2002; 21:abstr 329.10. Grothey A. Oxaliplatin-safety profile: neurotoxicity. Semin Oncol. 2003; 30(4 Suppl 15):5–13. PMID: 14523789.
Article11. Grothey A. Clinical management of oxaliplatin-associated neurotoxicity. Clin Colorectal Cancer. 2005; 5(Suppl 1):S38–S46. PMID: 15871765.
Article12. Cavaletti G, Bogliun G, Marzorati L, Zincone A, Piatti M, Colombo N, et al. Grading of chemotherapy-induced peripheral neurotoxicity using the Total Neuropathy Scale. Neurology. 2003; 61:1297–1300. PMID: 14610145.
Article13. Cleeland CS, Farrar JT, Hausheer FH. Assessment of cancer-related neuropathy and neuropathic pain. Oncologist. 2010; 15(Suppl 2):13–18. PMID: 20489192.
Article14. McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 2009; 8:10–16. PMID: 19139108.
Article15. Ruzzo A, Graziano F, Loupakis F, Rulli E, Canestrari E, Santini D, et al. Pharmacogenetic profiling in patients with advanced colorectal cancer treated with first-line FOLFOX-4 chemotherapy. J Clin Oncol. 2007; 25:1247–1254. PMID: 17401013.
Article16. Lecomte T, Landi B, Beaune P, Laurent-Puig P, Loriot MA. Glutathione S-transferase P1 polymorphism (Ile105Val) predicts cumulative neuropathy in patients receiving oxaliplatin-based chemotherapy. Clin Cancer Res. 2006; 12:3050–3056. PMID: 16707601.
Article17. Gamelin L, Capitain O, Morel A, Dumont A, Traore S, Anne le B, et al. Predictive factors of oxaliplatin neurotoxicity: the involvement of the oxalate outcome pathway. Clin Cancer Res. 2007; 13:6359–6368. PMID: 17975148.
Article18. Lee S, Won H, Son E, Lee J, Park S, Park J, et al. Genetic polymorphism associated with chronic neurotoxicity and recurrence in curatively-resected colon cancer patients receiving oxaliplatin-based adjuvant chemotherapy. J Clin Oncol. 2010; 28:abstr 3583.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Oxaliplatin-Induced Immune-Mediated Thrombocytopenia: A Case Report
- Oxaliplatin-induced Sudden Hearing Loss in a Patient with Pancreatic Cancer
- Leucovorin-induced Hypersensitivity Reaction in a Patient with Metastatic Colorectal Cancer Treated with Cetuximab Plus FOLFOX Chemotherapy: A Case Report
- A Prospective Study of Chronic Oxaliplatin-Induced Neuropathy in Patients with Colon Cancer: Long-Term Outcomes and Predictors of Severe Oxaliplatin-Induced Neuropathy
- Current Pharmacogenetic Approach for Oxaliplatin-induced Peripheral Neuropathy among Patients with Colorectal Cancer: A Systematic Review