Omalizumab Improves Quality of Life and Asthma Control in Chinese Patients With Moderate to Severe Asthma: A Randomized Phase III Study
- Affiliations
-
- 1State Key Laboratory of Respiratory Disease, The First Affiliated Hospital, Guangzhou Medical University, Guangzhou, China. nanshan@vip.163.com
- 2Institute of Respiratory Disease, First Hospital of China Medical University, Shenyang, China.
- 3Institute of Respiratory Disease, Xinqiao Hospital, Third Military Medical University, Chongqing, China.
- 4Respiratory Franchise, Beijing Novartis Pharma Co. Ltd., Beijing, China.
- 5IQS, Beijing Novartis Pharma Co. Ltd., Shanghai, China.
- 6Novartis Pharma AG, Basel, Switzerland.
- KMID: 2165915
- DOI: http://doi.org/10.4168/aair.2016.8.4.319
Abstract
- PURPOSE
Omalizumab is the preferred add-on therapy for patients with moderate-to-severe persistent allergic asthma and has demonstrated efficacy and safety in various ethnicities. This study evaluated the efficacy and safety of omalizumab in Chinese patients with moderate-to-severe allergic asthma.
METHODS
This randomized, double-blind, parallel-group, placebo-controlled, phase III study assessed lung function, quality of life, asthma control, and safety of omalizumab after 24-week therapy in Chinese patients (18-75 years of age).
RESULTS
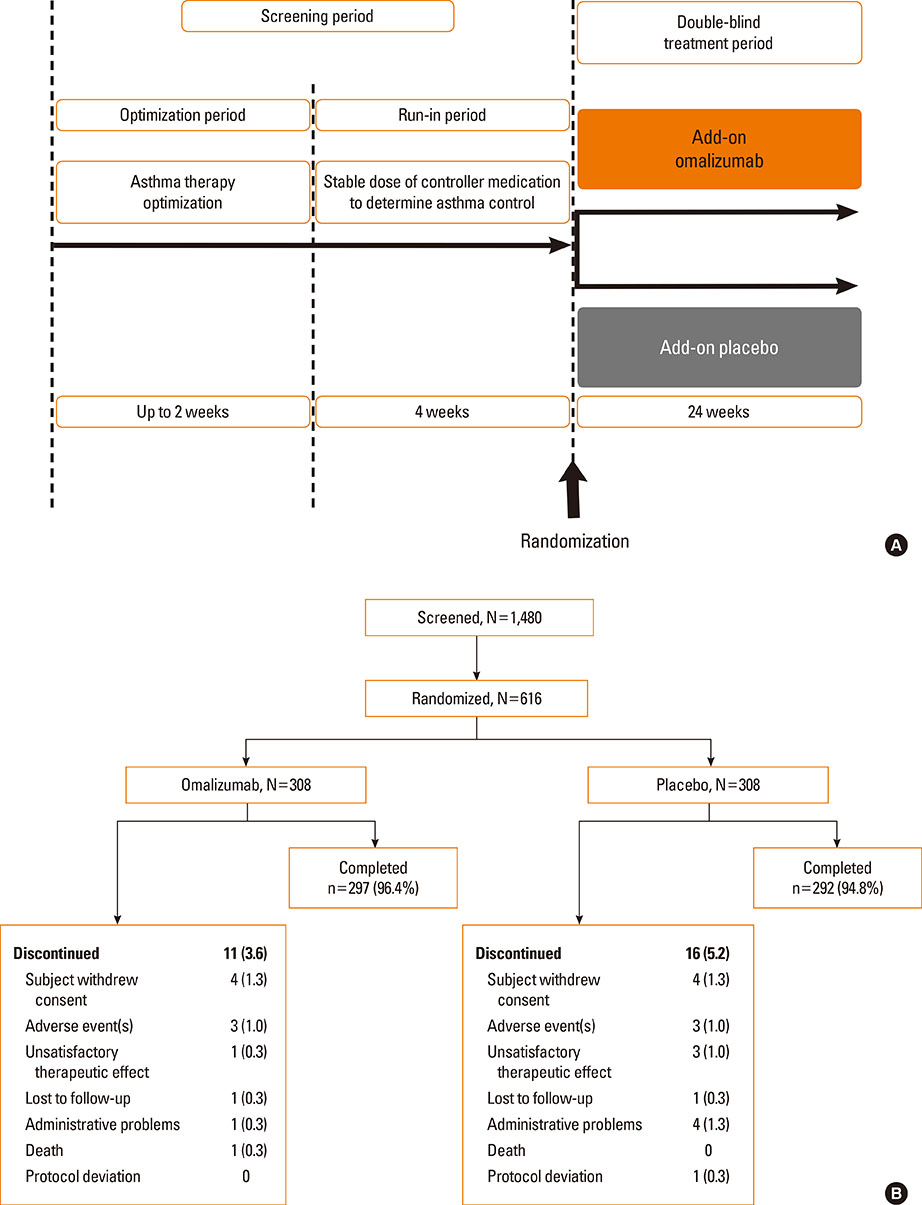
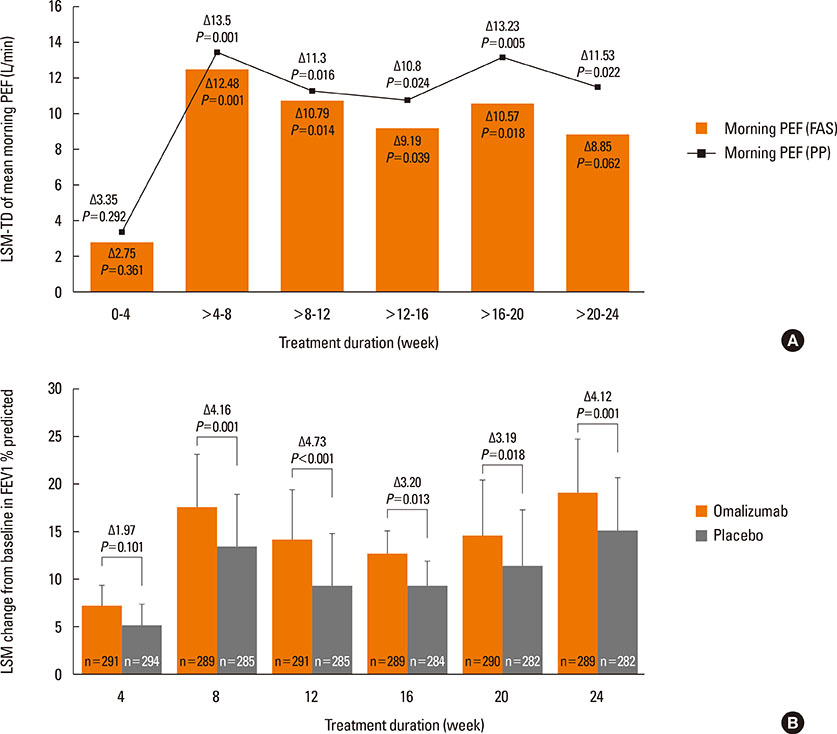
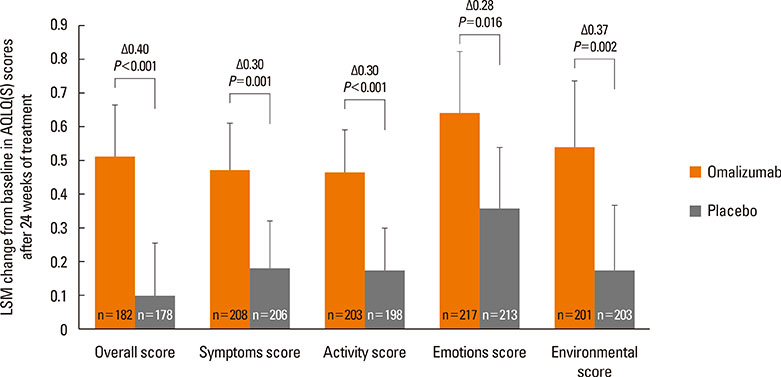
A total of 616 patients were randomized (1:1) to omalizumab or placebo. The primary endpoint, least squares mean treatment difference (LSM-TD) in morning peak expiratory flow (PEF) (omalizumab vs placebo), at Weeks >20-24 was 8.85 L/min (Full analysis set; P=0.062). Per-protocol analysis set showed significant improvements with LSM-TD of 11.53 L/min in mean mPEF at Weeks >20-24 (P=0.022). The FEV1 % predicted was significantly improved with omalizumab vs placebo from 8 to 24 weeks (after 24-week treatment: LSM-TD=4.12%; P=0.001). At Week 24, a higher proportion of omalizumab-treated patients achieved clinically relevant improvements in standardized AQLQ (58.2% vs 39.3%; LSM=0.51 vs 0.10; P<0.001) and ACQ (49.5% vs 35.5%; LSM=-0.51 vs -0.34; P=0.002) scores vs placebo. Total and nighttime symptom scores reduced significantly with omalizumab vs placebo (LSM-TD=-0.21, P=0.048 and -0.12, P=0.011, respectively). Although the study was not powered to study differences in exacerbation rates (P=0.097), exacerbations in winter months were less frequent in the omalizumab vs placebo group (2 vs 21). Adverse event and severe adverse event rates were comparable between omalizumab and placebo.
CONCLUSIONS
Omalizumab improves lung function, quality of life, and asthma control in Chinese patients with moderate-to-severe persistent allergic asthma and has a good safety profile.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Characteristics of Adult Severe Refractory Asthma in Korea Analyzed From the Severe Asthma Registry
Min-Hye Kim, Sang-Heon Kim, So-Young Park, Ga-Young Ban, Joo-Hee Kim, Jae-Woo Jung, Ji Yong Moon, Woo-Jung Song, Hyouk-Soo Kwon, Jae-Woo Kwon, Jae Hyun Lee, Hye-Ryun Kang, Jong-Sook Park, Tae-Bum Kim, Heung-Woo Park, Kwang-Ha Yoo, Yeon-Mok Oh, Young-Il Koh, An-Soo Jang, Byung-Jae Lee, Young-Joo Cho, Sang-Heon Cho, Hae-Sim Park, Choon-Sik Park, Ho Joo Yoon, You Sook Cho
Allergy Asthma Immunol Res. 2019;11(1):43-54. doi: 10.4168/aair.2019.11.1.43.Therapeutic Effect of Omalizumab in Severe Asthma: A Real-World Study in Korea
Ji-Ho Lee, Hyun Young Lee, Chang-Gyu Jung, Ga-Young Ban, Yoo Seob Shin, Young-Min Ye, Dong-Ho Nahm, Hae-Sim Park
Allergy Asthma Immunol Res. 2018;10(2):121-130. doi: 10.4168/aair.2018.10.2.121.
Reference
-
1. Holgate ST, Djukanović R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005; 35:408–416.2. Platts-Mills TA. The role of immunoglobulin E in allergy and asthma. Am J Respir Crit Care Med. 2001; 164:S1–S5.3. Ye YM, Kim SH, Hur GY, Kim JH, Park JW, Shim JJ, et al. Addition of montelukast to low-dose inhaled corticosteroid leads to fewer exacerbations in older patients than medium-dose inhaled corticosteroid monotherapy. Allergy Asthma Immunol Res. 2015; 7:440–448.4. Usmani OS. Small airways dysfunction in asthma: evaluation and management to improve asthma control. Allergy Asthma Immunol Res. 2014; 6:376–388.5. Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004; 59:469–478.6. Li J, Wang H, Chen Y, Zheng J, Wong GW, Zhong N. House dust mite sensitization is the main risk factor for the increase in prevalence of wheeze in 13- to 14-year-old schoolchildren in Guangzhou city, China. Clin Exp Allergy. 2013; 43:1171–1179.7. Li XY, Hu N, Huang ZJ, Jiang Y, Wu F. Mortality and death cause proportion of respiratory disease in China, 2004-2005. Zhonghua Yu Fang Yi Xue Za Zhi. 2010; 44:298–302.8. Asthma Workgroup. Chinese Thoracic Society. Chinese Societ of General Practitioners. Chinese guideline for the prevention and management of bronchial asthma (Primary Health Care Version). J Thorac Dis. 2013; 5:667–677.9. Chipps BE, Zeiger RS, Borish L, Wenzel SE, Yegin A, Hayden ML, et al. Key findings and clinical implications from the Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study. J Allergy Clin Immunol. 2012; 130:332–342.e10.10. López Tiro JJ, Contreras EA, del Pozo ME, Gómez Vera J, Larenas Linnemann D. Real life study of three years omalizumab in patients with difficult-to-control asthma. Allergol Immunopathol (Madr). 2015; 43:120–126.11. Song WJ, Cho SH. Challenges in the management of asthma in the elderly. Allergy Asthma Immunol Res. 2015; 7:431–439.12. Inführ D, Crameri R, Lamers R, Achatz G. Molecular and cellular targets of anti-IgE antibodies. Allergy. 2005; 60:977–985.13. Nam YH, Kim JH, Jin HJ, Hwang EK, Shin YS, Ye YM, et al. Effects of omalizumab treatment in patients with refractory chronic urticaria. Allergy Asthma Immunol Res. 2012; 4:357–361.14. Sullivan SD, Turk F. An evaluation of the cost-effectiveness of omalizumab for the treatment of severe allergic asthma. Allergy. 2008; 63:670–684.15. Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes-Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011; 154:573–582.16. Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005; 60:309–316.17. Niven R, Chung KF, Panahloo Z, Blogg M, Ayre G. Effectiveness of omalizumab in patients with inadequately controlled severe persistent allergic asthma: an open-label study. Respir Med. 2008; 102:1371–1378.18. Bousquet J, Siergiejko Z, Swiebocka E, Humbert M, Rabe KF, Smith N, et al. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy. 2011; 66:671–678.19. Braunstahl GJ, Chen CW, Maykut R, Georgiou P, Peachey G, Bruce J. The eXpeRience registry: the 'real-world' effectiveness of omalizumab in allergic asthma. Respir Med. 2013; 107:1141–1151.20. National Heart, Lung, and Blood Institute (US). Guidelines for the diagnosis and management of asthma (EPR-3) [Internet]. Bethesda (MD): National Heart, Lung, and Blood Institute;2007. 08. cited 2015 Jan 20. Available from: http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines.21. Barnes N, Menzies-Gow A, Mansur AH, Spencer D, Percival F, Radwan A, et al. Effectiveness of omalizumab in severe allergic asthma: a retrospective UK real-world study. J Asthma. 2013; 50:529–536.22. Cazzola M, Camiciottoli G, Bonavia M, Gulotta C, Ravazzi A, Alessandrini A, et al. Italian real-life experience of omalizumab. Respir Med. 2010; 104:1410–1416.23. Deschildre A, Marguet C, Salleron J, Pin I, Rittié JL, Derelle J, et al. Add-on omalizumab in children with severe allergic asthma: a 1-year real life survey. Eur Respir J. 2013; 42:1224–1233.24. D'Urzo AD, Wong J. Effectiveness of omalizumab in severe persistent asthma under real-life conditions. Can Fam Physician. 2014; 60:643–645.25. Novelli F, Latorre M, Vergura L, Caiaffa MF, Camiciottoli G, Guarnieri G, et al. Asthma control in severe asthmatics under treatment with omalizumab: a cross-sectional observational study in Italy. Pulm Pharmacol Ther. 2015; 31:123–129.26. Ohta K, Miyamoto T, Amagasaki T, Yamamoto M, Study G. 1304 Study Group. Efficacy and safety of omalizumab in an Asian population with moderate-to-severe persistent asthma. Respirology. 2009; 14:1156–1165.27. Ohta K, Yamamoto M, Sato N, Ikeda K, Miyamoto T. One year treatment with omalizumab is effective and well tolerated in Japanese Patients with moderate-to-severe persistent asthma. Allergol Int. 2010; 59:167–174.28. Schumann C, Kropf C, Wibmer T, Rüdiger S, Stoiber KM, Thielen A, et al. Omalizumab in patients with severe asthma: the XCLUSIVE study. Clin Respir J. 2012; 6:215–227.29. Subramaniam A, Al-Alawi M, Hamad S, O'Callaghan J, Lane SJ. A study into efficacy of omalizumab therapy in patients with severe persistent allergic asthma at a tertiary referral centre for asthma in Ireland. QJM. 2013; 106:631–634.30. Tajiri T, Niimi A, Matsumoto H, Ito I, Oguma T, Otsuka K, et al. Comprehensive efficacy of omalizumab for severe refractory asthma: a time-series observational study. Ann Allergy Asthma Immunol. 2014; 113:470–475.e2.31. Braunstahl GJ, Chlumský J, Peachey G, Chen CW. Reduction in oral corticosteroid use in patients receiving omalizumab for allergic asthma in the real-world setting. Allergy Asthma Clin Immunol. 2013; 9:47.32. Brusselle G, Michils A, Louis R, Dupont L, Van de Maele B, Delobbe A, et al. "Real-life" effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respir Med. 2009; 103:1633–1642.33. Johnston NW, Sears MR. Asthma exacerbations. 1: epidemiology. Thorax. 2006; 61:722–728.34. Wark PA, Gibson PG. Asthma exacerbations. 3: pathogenesis. Thorax. 2006; 61:909–915.35. Solèr M, Matz J, Townley R, Buhl R, O'Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001; 18:254–261.36. Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001; 108:184–190.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is Omalizumab a Problem-Solving Remedy in Severe Asthma?
- Treatment of Severe Asthma
- Written Asthma Action Plan Improves Asthma Control and the Quality of Life among Pediatric Asthma Patients in Malaysia: A Randomized Control Trial
- Real-life Efficacy of Omalizumab After 9 Years of Follow-up
- Omalizumab and unmet needs in severe asthma and allergic comorbidities in Japanese children