J Pathol Transl Med.
2016 May;50(3):181-189. 10.4132/jptm.2016.03.18.
Nuclear Expression of Hepatitis B Virus X Protein Is Associated with Recurrence of Early-Stage Hepatocellular Carcinomas: Role of Viral Protein in Tumor Recurrence
- Affiliations
-
- 1Department of Pathology, Seoul National University College of Medicine, Seoul, Korea. kblee@snuh.org
- 2Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2164594
- DOI: http://doi.org/10.4132/jptm.2016.03.18
Abstract
- BACKGROUND
Hepatitis B virus (HBV) plays well-known roles in tumorigenesis of hepatocellular carcinoma (HCC) in infected patients. However, HBV-associated protein status in tumor tissues and the relevance to tumor behavior has not been reported. Our study aimed to examine the expression of HBV-associated proteins in HCC and adjacent nontumorous tissue and their clinicopathologic implication in HCC patients.
METHODS
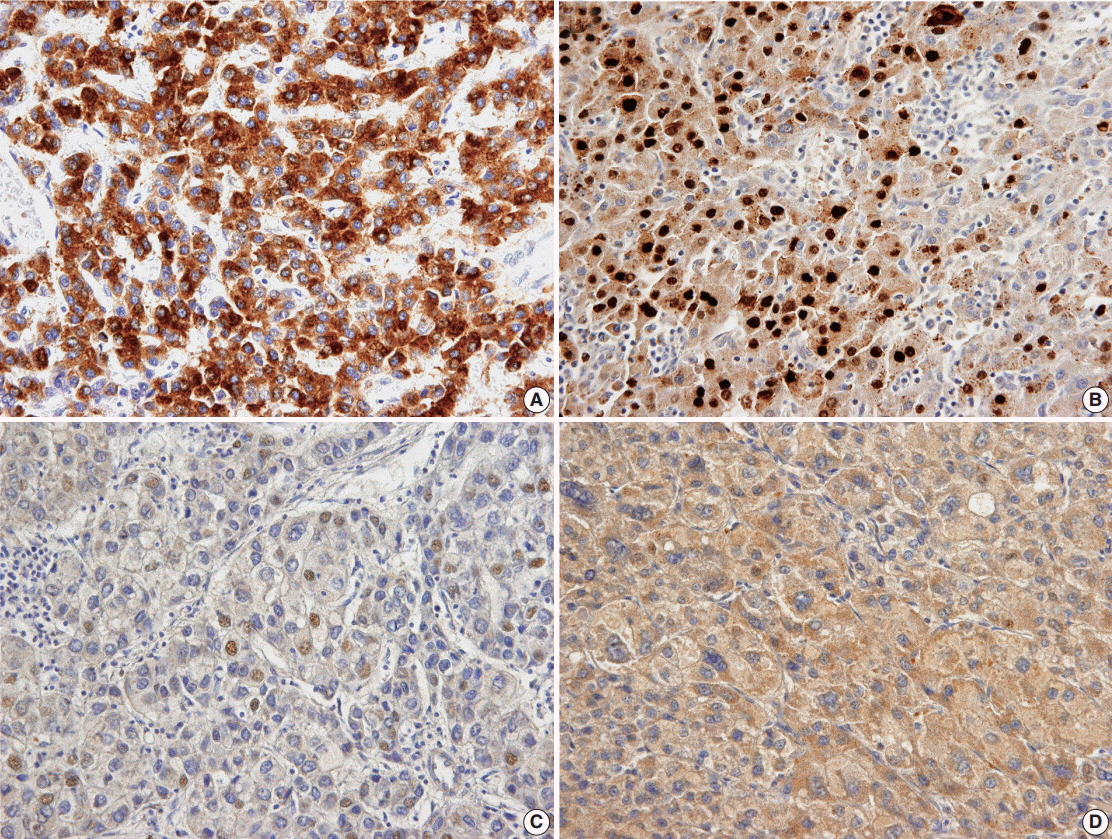
HBV surface antigen (HBsAg), HBV core antigen (HBcAg), and HBV X protein (HBx) were assessed in 328 HBV-associated HCCs and in 155 matched nontumorous tissues by immunohistochemistry staining.
RESULTS
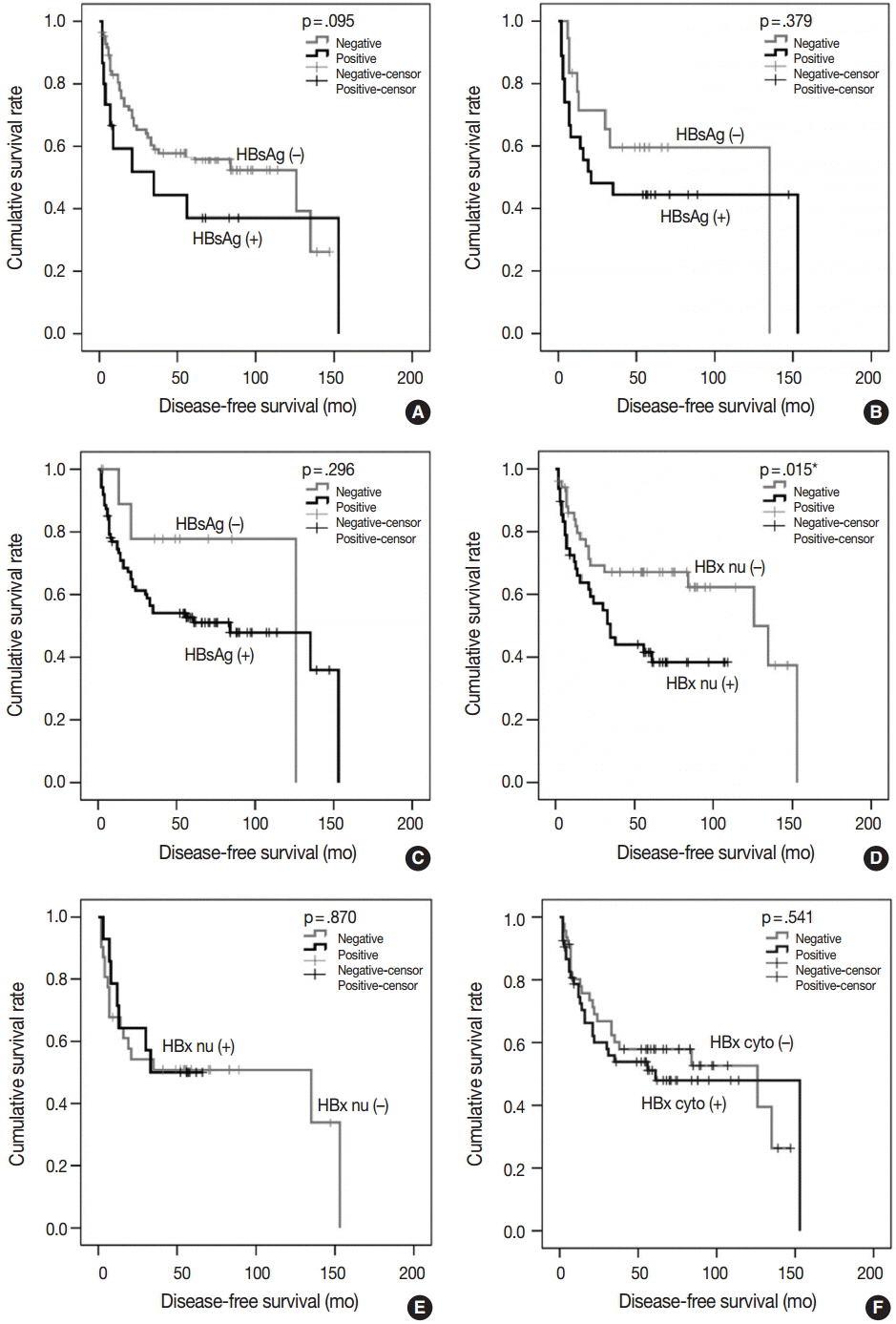
The positive rates of HBsAg and cytoplasmic HBx staining in tumor tissue were lower than those in nontumorous tissue (7.3% vs. 57.4%, p < .001; 43.4% vs. 81.3%, p < .001). Conversely, nuclear HBx was detected more frequently in tumors than in nontumorous tissue (52.1% vs. 30.3%, p < .001). HCCs expressing HBsAg, HBcAg, or cytoplasmic HBx had smaller size; lower Edmondson-Steiner (ES) nuclear grade, pT stage, and serum alpha-fetoprotein, and less angioinvasion than HCCs not expressing HBV-associated proteins. Exceptionally, nuclear HBx-positive HCCs showed higher ES nuclear grade and more frequent large-vessel invasion than did nuclear HBx-negative HCCs. In survival analysis, only nuclear HBx-positive HCCs had shorter disease-free survival than nuclear HBx-negative HCCs in pT1 and ES nuclear grade 1-2 HCC subgroup (median, 126 months vs. 35 months; p = .015).
CONCLUSIONS
Our data confirmed that expression of normal HBV-associated proteins generally decreases in tumor cells in comparison to nontumorous hepatocytes, with the exception of nuclear HBx, which suggests that nuclear HBx plays a role in recurrence of well-differentiated and early-stage HCCs.
Keyword
MeSH Terms
-
alpha-Fetoproteins
Antigens, Surface
Carcinogenesis
Carcinoma, Hepatocellular*
Cytoplasm
Disease-Free Survival
Hepatitis B Core Antigens
Hepatitis B Surface Antigens
Hepatitis B virus*
Hepatitis B*
Hepatitis*
Hepatocytes
Humans
Immunohistochemistry
Recurrence*
Antigens, Surface
Hepatitis B Core Antigens
Hepatitis B Surface Antigens
alpha-Fetoproteins
Figure
Reference
-
1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127:2893–917.
Article2. Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010; 42 Suppl 3:S206–14.
Article3. Brechot C, Jaffredo F, Lagorce D, et al. Impact of HBV, HCV and GBV-C/HGV on hepatocellular carcinomas in Europe: results of a European concerted action. J Hepatol. 1998; 29:173–83.4. Theise ND, Bodenheimer HC Jr, Ferrell LD. Acute and chronic viral hepatitis. In : Burt AD, Portmann BC, Ferrell LD, editors. MacSween’s pathology of the liver. 6th ed. Edinburgh: Churchill Livingstone Elsevier;2012. p. 361–401.5. Ng SA, Lee C. Hepatitis B virus X gene and hepatocarcinogenesis. J Gastroenterol. 2011; 46:974–90.
Article6. Goodman ZD, Terraciano LM, Wee A. Tumours and tumour-like lesions of the liver. In : Burt AD, Portmann B, Ferrell L, editors. MacSween’s pathology of the liver. Edinburgh: Churchill Livingstone Elsevier;2012. p. 761–851. 6th.7. Xia F, Lai EC, Lau WY, et al. High serum hyaluronic acid and HBV viral load are main prognostic factors of local recurrence after complete radiofrequency ablation of hepatitis B-related small hepatocellular carcinoma. Ann Surg Oncol. 2012; 19:1284–91.
Article8. Huang G, Yang Y, Shen F, et al. Early viral suppression predicts good postoperative survivals in patients with hepatocellular carcinoma with a high baseline HBV-DNA load. Ann Surg Oncol. 2013; 20:1482–90.
Article9. Yin J, Xie J, Liu S, et al. Association between the various mutations in viral core promoter region to different stages of hepatitis B, ranging of asymptomatic carrier state to hepatocellular carcinoma. Am J Gastroenterol. 2011; 106:81–92.
Article10. Tsai HW, Lin YJ, Lin PW, et al. A clustered ground-glass hepatocyte pattern represents a new prognostic marker for the recurrence of hepatocellular carcinoma after surgery. Cancer. 2011; 117:2951–60.
Article11. Kremsdorf D, Soussan P, Paterlini-Brechot P, Brechot C. Hepatitis B virus-related hepatocellular carcinoma: paradigms for viral-related human carcinogenesis. Oncogene. 2006; 25:3823–33.
Article12. Zhu M, London WT, Duan LX, Feitelson MA. The value of hepatitis B x antigen as a prognostic marker in the development of hepatocellular carcinoma. Int J Cancer. 1993; 55:571–6.
Article13. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–4.
Article14. The general rules for the clinical and pathological study of primary liver cancer. Liver Cancer Study Group of Japan. Jpn J Surg. 1989; 19:98–129.15. Shiota G, Oyama K, Udagawa A, et al. Occult hepatitis B virus infection in HBs antigen-negative hepatocellular carcinoma in a Japanese population: involvement of HBx and p53. J Med Virol. 2000; 62:151–8.
Article16. Keasler VV, Hodgson AJ, Madden CR, Slagle BL. Hepatitis B virus HBx protein localized to the nucleus restores HBx-deficient virus replication in HepG2 cells and in vivo in hydrodynamically-injected mice. Virology. 2009; 390:122–9.
Article17. Sung WK, Lu Y, Lee CW, Zhang D, Ronaghi M, Lee CG. Deregulated direct targets of the hepatitis B virus (HBV) protein, HBx, identified through chromatin immunoprecipitation and expression microarray profiling. J Biol Chem. 2009; 284:21941–54.
Article18. Ou DP, Tao YM, Tang FQ, Yang LY. The hepatitis B virus X protein promotes hepatocellular carcinoma metastasis by upregulation of matrix metalloproteinases. Int J Cancer. 2007; 120:1208–14.
Article19. Xie H, Song J, Liu K, et al. The expression of hypoxia-inducible factor-1alpha in hepatitis B virus-related hepatocellular carcinoma: correlation with patients’ prognosis and hepatitis B virus X protein. Dig Dis Sci. 2008; 53:3225–33.20. Xia L, Huang W, Tian D, et al. Upregulated FoxM1 expression induced by hepatitis B virus X protein promotes tumor metastasis and indicates poor prognosis in hepatitis B virus-related hepatocellular carcinoma. J Hepatol. 2012; 57:600–12.
Article21. Liu H, Xu L, He H, et al. Hepatitis B virus X protein promotes hepatoma cell invasion and metastasis by stabilizing Snail protein. Cancer Sci. 2012; 103:2072–81.
Article22. Zhang X, Dong N, Zhang H, You J, Wang H, Ye L. Effects of hepatitis B virus X protein on human telomerase reverse transcriptase expression and activity in hepatoma cells. J Lab Clin Med. 2005; 145:98–104.
Article23. Su CW, Chiou YW, Tsai YH, et al. The influence of hepatitis B viral load and pre-S deletion mutations on post-operative recurrence of hepatocellular carcinoma and the tertiary preventive effects by anti-viral therapy. PLoS One. 2013; 8:e66457.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intracellular Antibody Fragment Against Hepatitis B Virus X Protein Does Not Inhibit Viral Replication
- Management of viral hepatitis in patients with hepatocellular carcinoma
- High Expression of Ribonucleotide Reductase Subunit M2 Correlates with Poor Prognosis of Hepatocellular Carcinoma
- Immunohistochemieal Study of Expression of nm23 and CD44 Protein in
- Hepatitis B Precore Protein: Pathogenic Potential and Therapeutic Promise