Int J Stem Cells.
2016 May;9(1):152-162. 10.15283/ijsc.2016.9.1.152.
Moderate Hypoxia Exhibits Increased Endothelial Progenitor Vessel-forming Ability However Gestational Diabetes Caused to Impede Compensatory Defense Reaction
- Affiliations
-
- 1Department of Pharmacology, School of Pharmacy, Girne American University, Girne, North Cyprus via Mersin 10, Turkey. denizdincer@gau.edu.tr
- 2Department of Clinical Pharmacy, School of Pharmacy, Girne American University, Girne, North Cyprus via Mersin 10, Turkey.
- 3Department of Pharmacology, School of Medicine, Girne American University, Girne, North Cyprus via Mersin 10, Turkey.
- KMID: 2164172
- DOI: http://doi.org/10.15283/ijsc.2016.9.1.152
Abstract
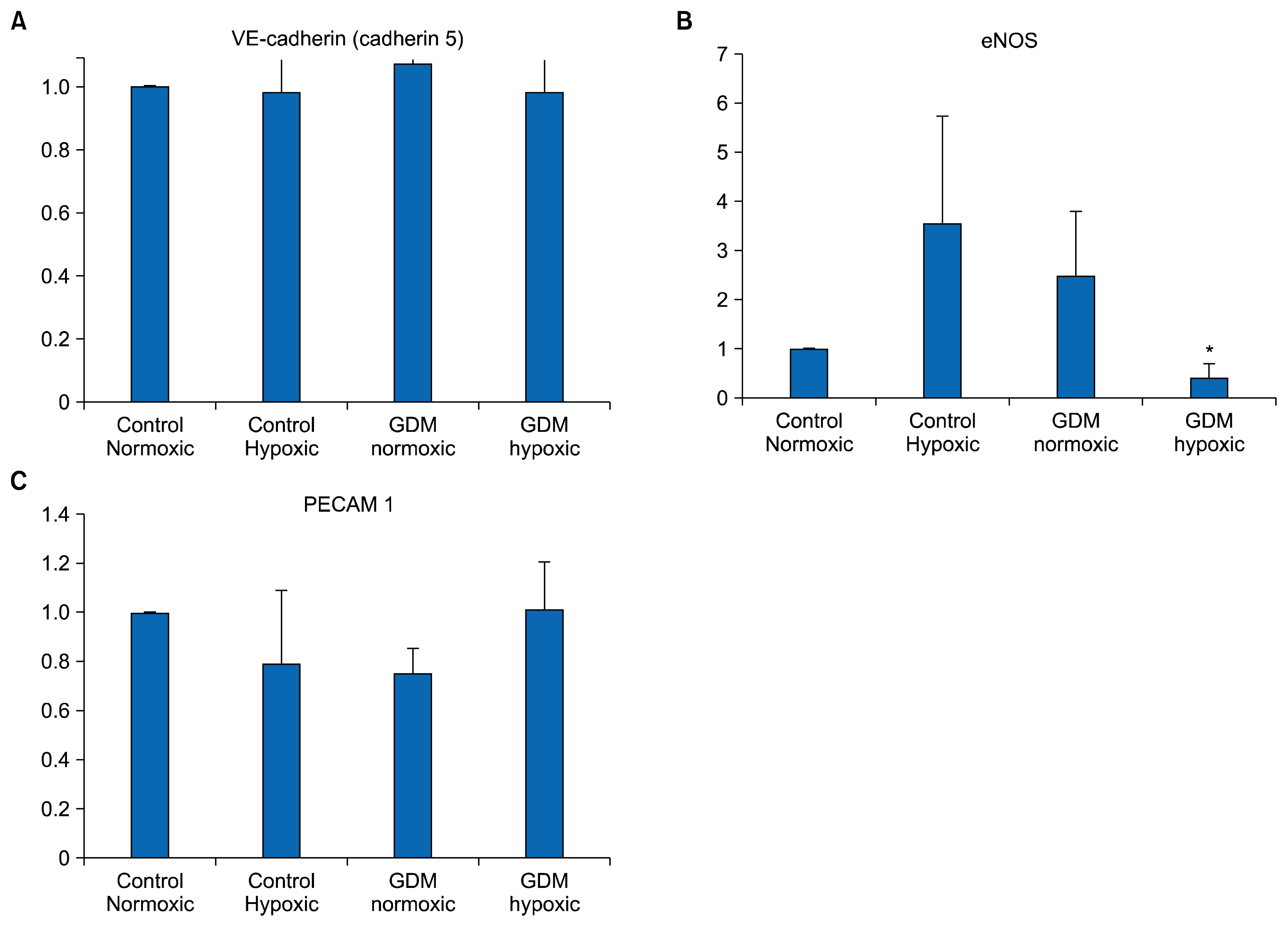
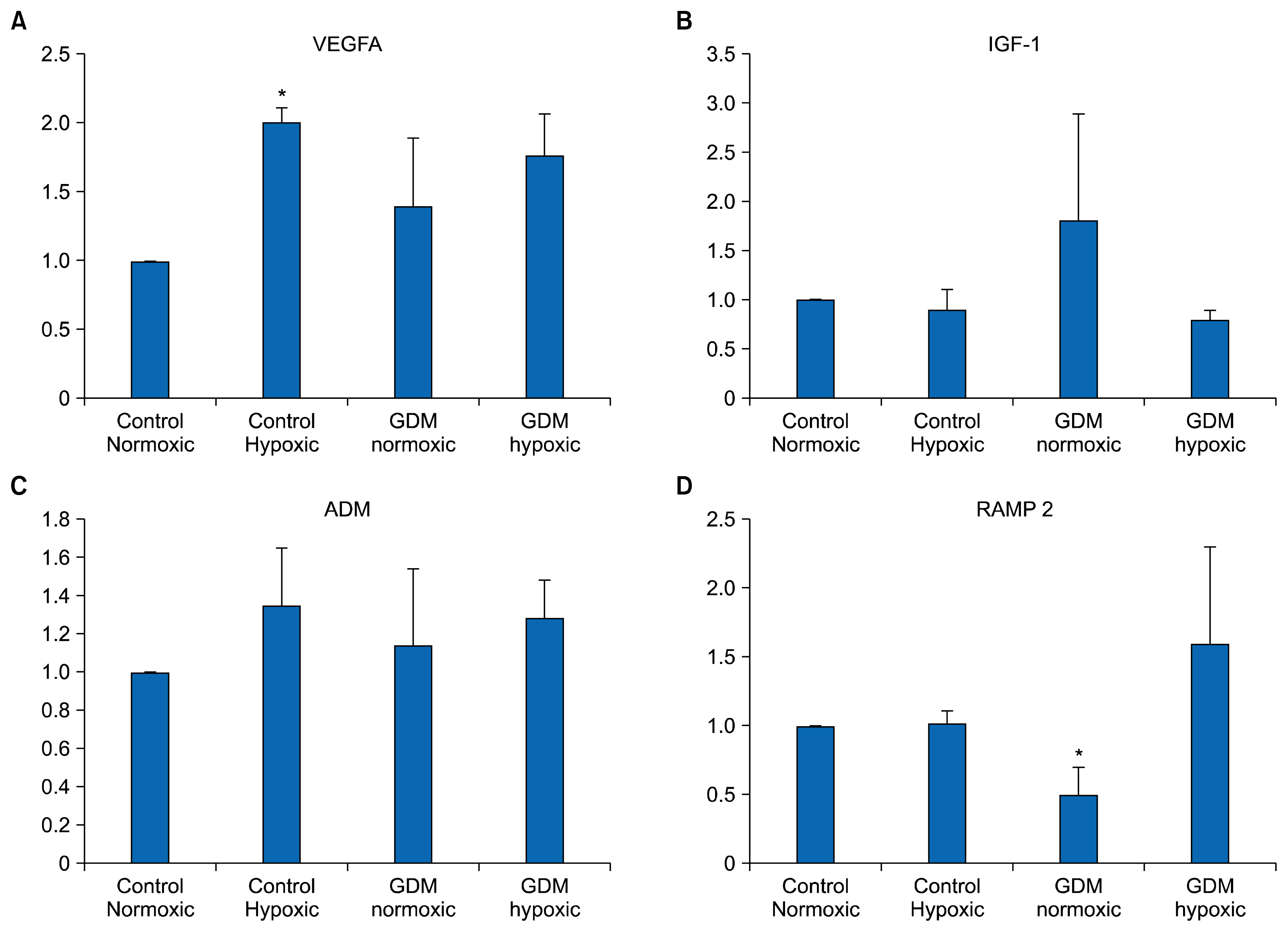
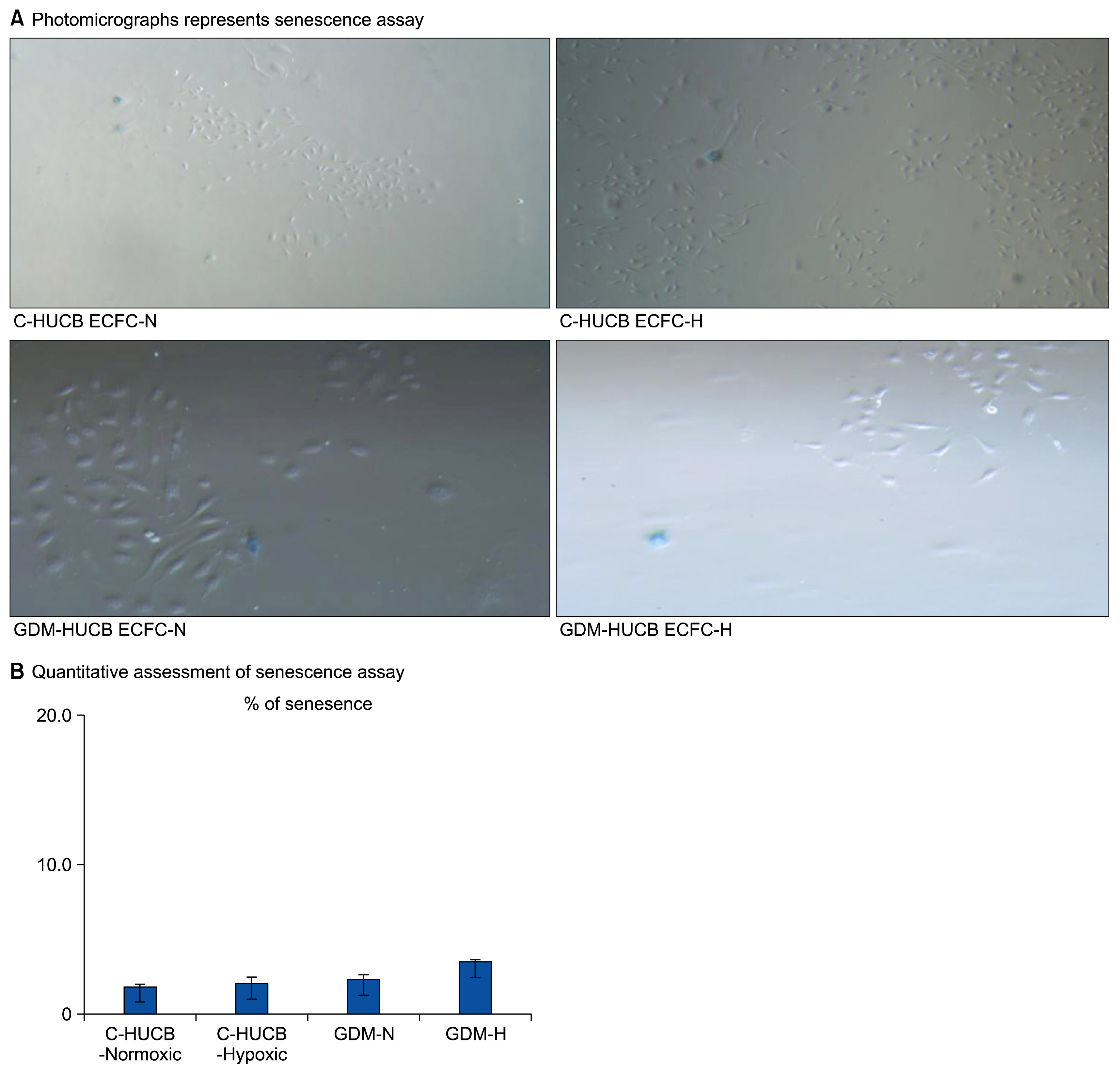
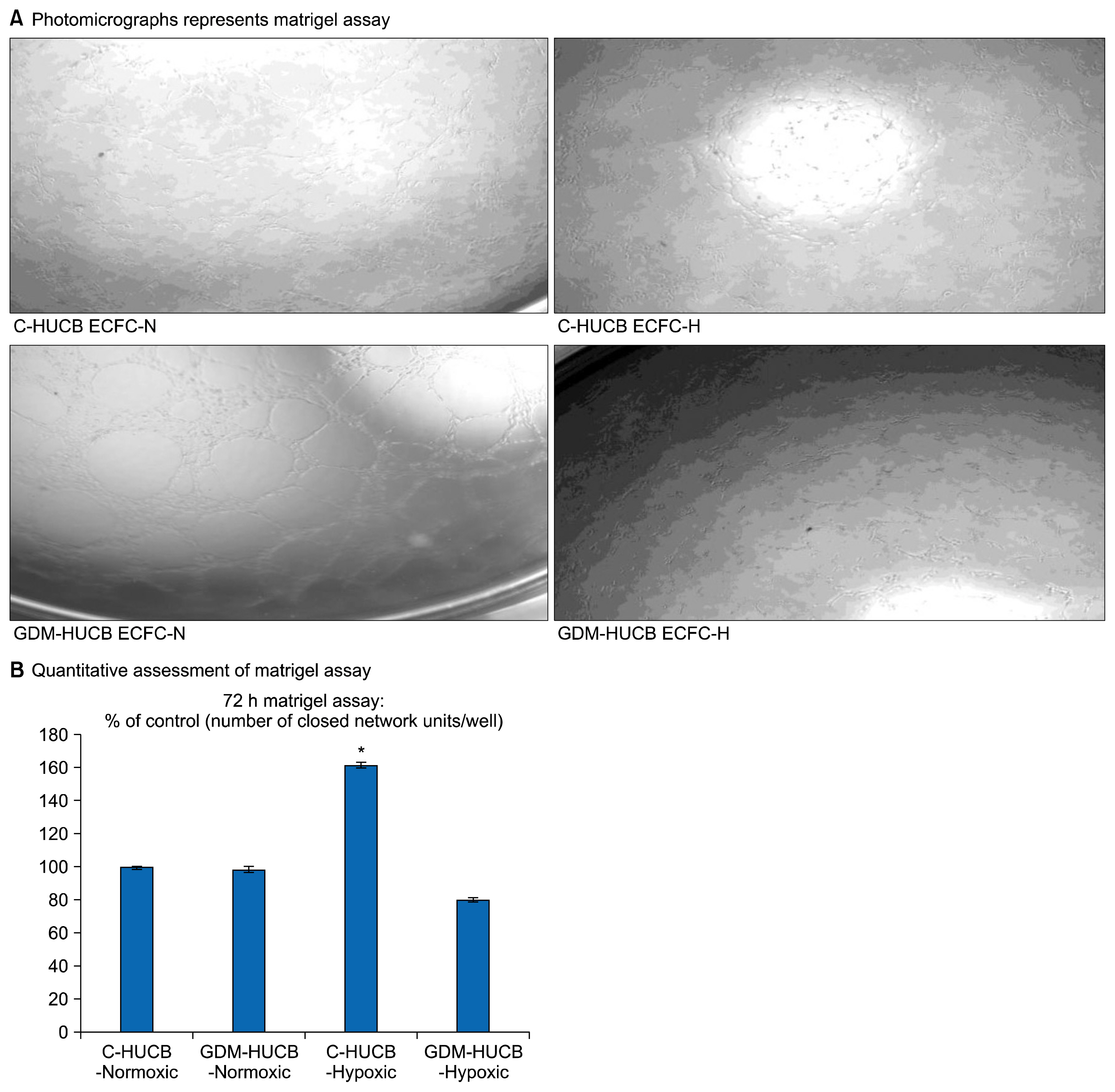
- Endothelium represents a defense barrier and responds and integrates neuro humoral stimulus which describes as a compensatory mechanism. Endothelium formed with endothelial cells (ECs) and their progenitors. Endothelial progenitor cells (EPCs) represent minor subpopulation of mononuclear cells in the blood. During acute hypoxia, larger amount of EPCs mobilize into the peripheral blood and they directly contribute revascularization process. One of the subtypes of EPC is termed endothelial colony forming cells (ECFCs) which they possess de novo vessel-forming ability. The present study aims to investigate the role of hypoxia in EPCs functional and vessel-forming ability. Furthermore, it was investigated whether fetal exposure to a diabetic intrauterine environment influence EPCs adaptation ability. Human umbilical cord blood (HUCB) derived ECFCs were selected in all experimental procedures obtained from normal and gestational diabetes mellitus (GDM) subjects via in vitro cell culture methods. Early passage (<5) HUCB ECFCs obtain from GDM (n; 5) and control (n; 5) subjects were cultured with plates pre-coated with collagen in vitro 72 h hypoxic as well as normoxic condition. Endothelial, angiogenic and hypoxia associated gene specific primers designed to perform Real-time PCR. Senescenes assay conducted onto HUCB ECFCs to investigate their functional clonogenic ability. To quantify their vessel forming ability matrigel assay was applied. These data demonstrates that moderate hypoxia results increased vessel-forming ability and VEGFA expression in HUCB ECFCs obtained from control subjects. However, GDM caused to impede compensatory defense reaction against hypoxia which observed in control subjects. Thus, it illuminates beneficial information related future therapeutic modalities.
MeSH Terms
Figure
Reference
-
References
1. Prasain N, Lee MR, Vemula S, Meador JL, Yoshimoto M, Ferkowicz MJ, Fett A, Gupta M, Rapp BM, Saadatzadeh MR, Ginsberg M, Elemento O, Lee Y, Voytik-Harbin SL, Chung HM, Hong KS, Reid E, O’Neill CL, Medina RJ, Stitt AW, Murphy MP, Rafii S, Broxmeyer HE, Yoder MC. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nat Biotechnol. 2014; 32:1151–1157. DOI: 10.1038/nbt.3048. PMID: 25306246. PMCID: 4318247.
Article2. Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997; 275:964–967. DOI: 10.1126/science.275.5302.964. PMID: 9020076.
Article3. Ghani U, Shuaib A, Salam A, Nasir A, Shuaib U, Jeerakathil T, Sher F, O’Rourke F, Nasser AM, Schwindt B, Todd K. Endothelial progenitor cells during cerebrovascular disease. Stroke. 2005; 36:151–153. DOI: 10.1161/01.STR.0000149944.15406.16.
Article4. Liew A, Barry F, O’Brien T. Endothelial progenitor cells: diagnostic and therapeutic considerations. Bioessays. 2006; 28:261–270. DOI: 10.1002/bies.20372. PMID: 16479582.
Article5. Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007; 109:1801–1809. DOI: 10.1182/blood-2006-08-043471.
Article6. Kim J, Shapiro L, Flynn A. The clinical application of mesenchymal stem cells and cardiac stem cells as a therapy for cardiovascular disease. Pharmacol Ther. 2015; 151:8–15. DOI: 10.1016/j.pharmthera.2015.02.003. PMID: 25709098.
Article7. Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004; 53:195–199. DOI: 10.2337/diabetes.53.1.195.8. Imanishi T, Hano T, Matsuo Y, Nishio I. Oxidized low-density lipoprotein inhibits vascular endothelial growth factor-induced endothelial progenitor cell differentiation. Clin Exp Pharmacol Physiol. 2003; 30:665–670. DOI: 10.1046/j.1440-1681.2003.03894.x. PMID: 12940886.
Article9. Imanishi T, Moriwaki C, Hano T, Nishio I. Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential hypertension. J Hypertens. 2005; 23:1831–1837. DOI: 10.1097/01.hjh.0000183524.73746.1b. PMID: 16148606.
Article10. Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003; 348:593–600. DOI: 10.1056/NEJMoa022287. PMID: 12584367.
Article11. Dincer UD. Fetal exposure to a diabetic intrauterine environment resulted in a failure of cord blood endothelial progenitor cell adaptation against chronic hypoxia. Stem Cells Cloning. 2014; 8:1–14.
Article12. Blue EK, DiGiuseppe R, Derr-Yellin E, Acosta JC, Pay SL, Hanenberg H, Schellinger MM, Quinney SK, Mund JA, Case J, Haneline LS. Gestational diabetes induces alterations in the function of neonatal endothelial colony-forming cells. Pediatr Res. 2014; 75:266–272. DOI: 10.1038/pr.2013.224. PMCID: 3944713.
Article13. Yoder MC. Cord blood banking and transplantation: advances and controversies. Curr Opin Pediatr. 2014; 26:163–168. DOI: 10.1097/MOP.0000000000000065. PMID: 24553632.14. Timmermans F, Plum J, Yöder MC, Ingram DA, Vandekerckhove B, Case J. Endothelial progenitor cells: identity defined? J Cell Mol Med. 2009; 13:87–102. DOI: 10.1111/j.1582-4934.2008.00598.x.
Article15. Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007; 21:1141–1149. DOI: 10.1038/sj.leu.2404676. PMID: 17392816.
Article16. Paternotte E, Gaucher C, Labrude P, Stoltz JF, Menu P. Review: behaviour of endothelial cells faced with hypoxia. Biomed Mater Eng. 2008; 18:295–299. PMID: 19065037.
Article17. Hwang HS, Maeng YS, Kim YH, Kwon YG, Park YW, Kim IK. Nestin expression during differentiation of fetal endothelial progenitor cells and hypoxic culture of human umbilical vein endothelial cells. Acta Obstet Gynecol Scand. 2008; 87:643–651. DOI: 10.1080/00016340802085326. PMID: 18568464.
Article18. Ingram DA, Lien IZ, Mead LE, Estes M, Prater DN, Derr-Yellin E, DiMeglio LA, Haneline LS. In vitro hyperglycemia or a diabetic intrauterine environment reduces neonatal endothelial colony-forming cell numbers and function. Diabetes. 2008; 57:724–731. DOI: 10.2337/db07-1507.
Article19. Gavard J. Endothelial permeability and VE-cadherin: a wacky comradeship. Cell Adh Migr. 2013; 7:455–461. DOI: 10.4161/cam.27330.20. Rijcken E, Mennigen RB, Schaefer SD, Laukoetter MG, Anthoni C, Spiegel HU, Bruewer M, Senninger N, Krieglstein CF. PECAM-1 (CD 31) mediates transendothelial leukocyte migration in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2007; 293:G446–G452. DOI: 10.1152/ajpgi.00097.2007. PMID: 17510197.
Article21. Jafarifar F, Yao P, Eswarappa SM, Fox PL. Repression of VEGFA by CA-rich element-binding microRNAs is modulated by hnRNP L. EMBO J. 2011; 30:1324–1334. DOI: 10.1038/emboj.2011.38. PMID: 21343907. PMCID: 3094116.
Article22. Leung A, Ciau-Uitz A, Pinheiro P, Monteiro R, Zuo J, Vyas P, Patient R, Porcher C. Uncoupling VEGFA functions in arteriogenesis and hematopoietic stem cell specification. Dev Cell. 2013; 24:144–158. DOI: 10.1016/j.devcel.2012.12.004. PMID: 23318133. PMCID: 3560039.
Article23. Gombos A, Metzger-Filho O, Dal Lago L, Awada-Hussein A. Clinical development of insulin-like growth factor receptor--1 (IGF-1R) inhibitors: at the crossroad? Invest New Drugs. 2012; 30:2433–2442. DOI: 10.1007/s10637-012-9811-0. PMID: 22415797. PMCID: 3484277.
Article24. Wong HK, Tang F, Cheung TT, Cheung BM. Adrenomedullin and diabetes. World J Diabetes. 2014; 5:364–371. DOI: 10.4239/wjd.v5.i3.364. PMID: 24936257. PMCID: 4058740.
Article25. Larráyoz IM, Martínez-Herrero S, García-Sanmartín J, Ochoa-Callejero L, Martínez A. Adrenomedullin and tumour microenvironment. J Transl Med. 2014; 12:339. DOI: 10.1186/s12967-014-0339-2. PMID: 25475159. PMCID: 4272513.
Article26. Dincer UD, Araiza AG, Knudson JD, Molina PE, Tune JD. Sensitization of coronary alpha-adrenoceptor vasoconstriction in the prediabetic metabolic syndrome. Microcirculation. 2006; 13:587–595. DOI: 10.1080/10739680600885228. PMID: 16990217.
Article27. Dincer UD, Araiza A, Knudson JD, Shao CH, Bidasee KR, Tune JD. Dysfunction of cardiac ryanodine receptors in the metabolic syndrome. J Mol Cell Cardiol. 2006; 41:108–114. DOI: 10.1016/j.yjmcc.2006.04.018. PMID: 16793060.
Article28. Dincer UD. Cardiac β-adrenoceptor expression is markedly depressed in Ossabaw swine model of cardiometabolic risk. Int J Gen Med. 2011; 4:493–499. DOI: 10.2147/IJGM.S18175.29. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408. DOI: 10.1006/meth.2001.1262.
Article30. Dincer UD. Human endothelial progenitor cell application in vascular diseases seen in metabolic syndrome. J Metabolic Synd. 2015; 4:e115. DOI: 10.4172/2167-0943.1000e115.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Y-27632, a Rho-associated Kinase Inhibitor, on Human Corneal Endothelial Cells Cultured by Isolating Human Corneal Endothelial Progenitor Cells
- Regular Exercise Training Increases the Number of Endothelial Progenitor Cells and Decreases Homocysteine Levels in Healthy Peripheral Blood
- Adult Stem Cells: Beyond Regenerative Tool, More as a Bio-Marker in Obesity and Diabetes
- Negative-pressure wound therapy induces endothelial progenitor cell mobilization in diabetic patients with foot infection or skin defects
- Comparative Evaluation for Potential Differentiation of Endothelial Progenitor Cells and Mesenchymal Stem Cells into Endothelial-Like Cells