J Vet Sci.
2015 Mar;16(1):1-9. 10.4142/jvs.2015.16.1.1.
beta-irradiation (166Ho patch)-induced skin injury in mini-pigs: effects on NF-kappaB and COX-2 expression in the skin
- Affiliations
-
- 1Research Center, Dongnam Institute of Radiological and Medical Sciences, Busan 619-953, Korea. jskim@dirams.re.kr
- 2Department of Dermatology, Korea Institute of Radiological and Medical Sciences, Seoul 139-706, Korea.
- 3Department of Laboratory of Radiation Exposure and Therapeutics, Korea Institute of Radiological and Medical Sciences, Seoul 139-706, Korea.
- 4Department Nuclear Medicine, Korea Institute of Radiological and Medical Sciences, Seoul 139-706, Korea. smlim328@kirams.re.kr
- 5College of Veterinary Medicine, Chonnam National University, Gwangju 500-757, Korea.
- KMID: 2160814
- DOI: http://doi.org/10.4142/jvs.2015.16.1.1
Abstract
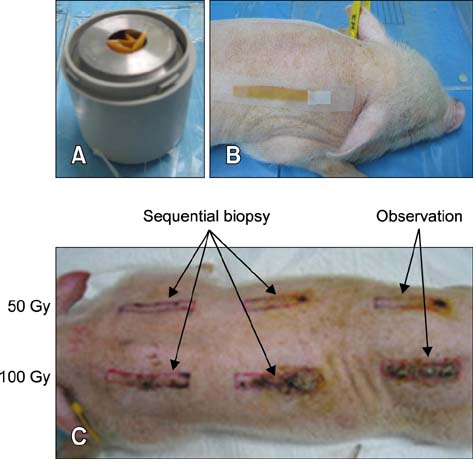
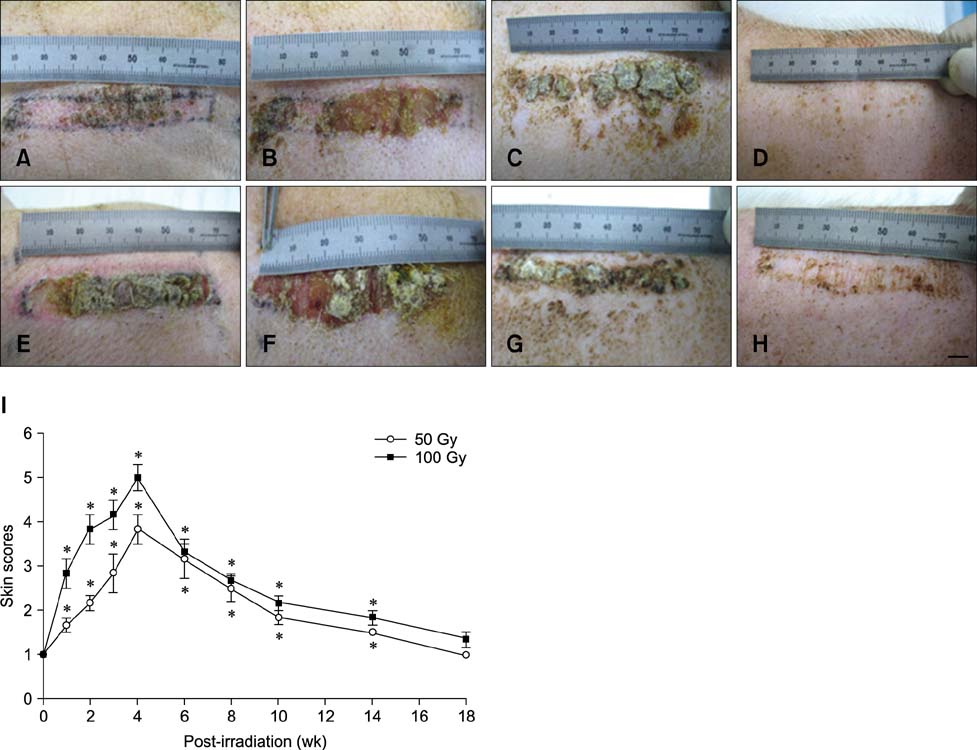
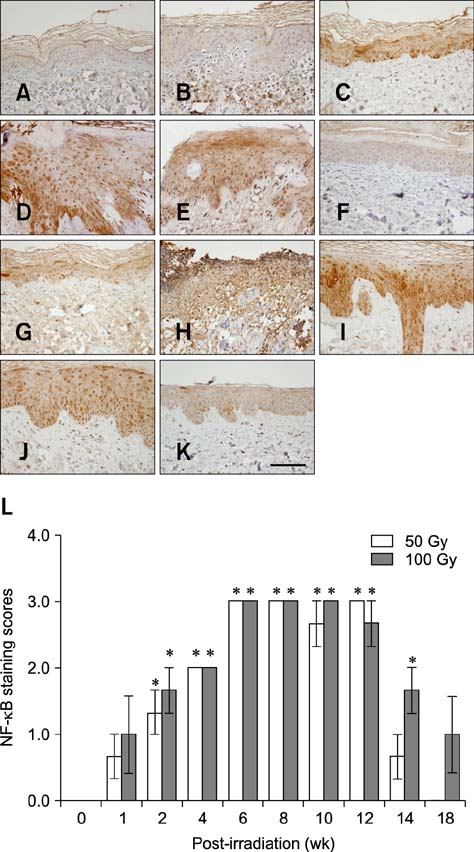
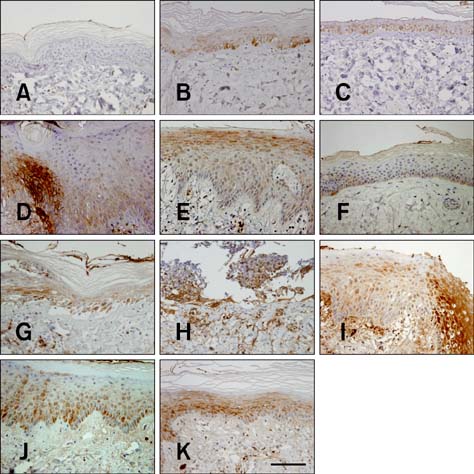
- In the present study, the detrimental effect of beta-emission on pig skin was evaluated. Skin injury was modeled in mini-pigs by exposing the animals to 50 and 100 Gy of beta-emission delivered by 166Ho patches. Clinicopathological and immunohistochemical changes in exposed skin were monitored for 18 weeks after beta-irradiation. Radiation induced desquamation at 2~4 weeks and gradual repair of this damage was evident 6 weeks after irradiation. Changes in basal cell density and skin depth corresponded to clinically relevant changes. Skin thickness began to decrease 1 week after irradiation, and the skin was thinnest 4 weeks after irradiation. Skin thickness increased transiently during recovery from irradiation-induced skin injury, which was evident 6~8 weeks after irradiation. Epidermal expression of nuclear factor-kappa B (NF-kappaB) differed significantly between the untreated and irradiated areas. One week after irradiation, cyclooxygenase-2 (COX-2) expression was mostly limited to the basal cell layer and scattered among these cells. High levels of COX-2 expression were detected throughout the full depth of the skin 4 weeks after irradiation. These findings suggest that NF-kappaB and COX-2 play roles in epidermal cell regeneration following beta-irradiation of mini-pig skin.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Therapeutic effect of topical application of curcumin during treatment of radiation burns in a mini-pig model
Joongsun Kim, Sunhoo Park, Byung-Suk Jeon, Won-Seok Jang, Sun-Joo Lee, Yeonghoon Son, Kyung-Jin Rhim, Soong In Lee, Seung-Sook Lee
J Vet Sci. 2016;17(4):435-444. doi: 10.4142/jvs.2016.17.4.435.
Reference
-
1. Abd-El-Aleem SA, Ferguson MWJ, Appleton I, Bhowmick A, McCollum CN, Ireland GW. Expression of cyclooxygenase isoforms in normal human skin and chronic venous ulcers. J Pathol. 2001; 195:616–623.
Article2. Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995; 31:1171–1185.
Article3. Athar M, An KP, Morel KD, Kim AL, Aszterbaum M, Longley J, Epstein EH Jr, Bickers DR. Ultraviolet B (UVB)-induced COX-2 expression in murine skin: an immunohistochemical study. Biochem Biophys Res Commun. 2001; 280:1042–1047.
Article4. Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J Clin Invest. 2001; 107:241–246.
Article5. Barabanova AV. Significance of beta-radiation skin burns in Chernobyl patients for the theory and practice of radiopathology. Vojnosanit Pregl. 2006; 63:477–480.
Article6. Brach MA, Hass R, Sherman ML, Gunji H, Weichselbaum R, Kufe D. Ionizing radiation induces expression and binding activity of the nuclear factor κB. J Clin Invest. 1991; 88:691–695.
Article7. Clark RAF, Ashcroft GS, Spencer MJ, Larjava H, Ferguson MWJ. Re-epithelialization of normal human excisional wounds is associated with a switch from αvβ5 to αvβ6 integrins. Br J Dermatol. 1996; 135:46–51.
Article8. Egan LJ, Eckmann L, Greten FR, Chae S, Li ZW, Myhre GM, Robine S, Karin M, Kagnoff MF. IκB-kinaseβ-dependent NF-κB activation provides radioprotection to the intestinal epithelium. Proc Natl Acad Sci U S A. 2004; 101:2452–2457.
Article9. Hei TK. Cyclooxygenase-2 as a signaling molecule in radiation-induced bystander effect. Mol Carcinog. 2006; 45:455–460.
Article10. Hoashi T, Okochi H, Kadono T, Tamaki K, Nishida M, Futami S, Maekawa K. A case of acute radiation syndrome from the dermatological aspect. Br J Dermatol. 2008; 158:597–602.
Article11. Hopewell JW. Mechanisms of the action of radiation on skin and underlying tissues. Br J Radiol Suppl. 1986; 19:39–47.12. Hopewell JW. The skin: its structure and response to ionizing radiation. Int J Radiat Biol. 1990; 57:751–773.
Article13. Hopewell JW, Sieber VK, Heryet JC, Wells J, Charles MW. Dose- and source-size-related changes in the late response of pig skin to irradiation with single doses of beta radiation from sources of differing energy. Radiat Res. 1993; 133:303–311.
Article14. Kumar S, Kolozsvary A, Kohl R, Lu M, Brown S, Kim JH. Radiation-induced skin injury in the animal model of scleroderma: implications for post-radiotherapy fibrosis. Radiat Oncol. 2008; 3:40.
Article15. Lee JD, Park KK, Lee MG, Kim EH, Rhim KJ, Lee JT, Yoo HS, Kim YM, Park KB, Kim JR. Radionuclide therapy of skin cancers and Bowen's disease using a specially designed skin patch. J Nucl Med. 1997; 38:697–702.16. Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997; 276:75–81.
Article17. Meng A, Yu T, Chen G, Brown SA, Wang Y, Thompson JS, Zhou D. Cellular origin of ionizing radiation-induced NF-κB activation in vivo and role of NF-κB in ionizing radiation-induced lymphocyte apoptosis. Int J Radiat Biol. 2003; 79:849–861.
Article18. Mettler FA Jr, Gus'kova AK, Gusev I. Health effects in those with acute radiation sickness from the Chernobyl accident. Health Phys. 2007; 93:462–469.
Article19. Morris GM, Hamlet R, Hopewell JW. The cell kinetics of the epidermis and follicular epithelium of the rat: variations with age and body site. Cell Tissue Kinet. 1989; 22:213–222.
Article20. Morris GM, Hopewell JW. Cell kinetic changes in the follicular epithelium of pig skin after irradiation with single and fractionated doses of X rays. Br J Radiol. 1989; 62:41–47.
Article21. Peel DM, Hopewell JW, Wells J, Charles MW. Nonstochastic effects of different energy β emitters on pig skin. Radiat Res. 1984; 99:372–382.22. Randall K, Coggle JE. Long-term expression of transforming growth factor TGF β1 in mouse skin after localized β-irradiation. Int J Radiat Biol. 1996; 70:351–360.
Article23. Rundhaug JE, Fischer SM. Cyclo-oxygenase-2 plays a critical role in UV-induced skin carcinogenesis. Photochem Photobiol. 2008; 84:322–329.
Article24. Schmedtje JF Jr, Ji YS, Liu WL, DuBois RN, Runge MS. Hypoxia induces cyclooxygenase-2 via the NF-κB p65 transcription factor in human vascular endothelial cells. J Biol Chem. 1997; 272:601–608.
Article25. Scholz K, Fürstenberger G, Müller-Decker K, Marks F. Differential expression of prostaglandin-H synthase isoenzymes in normal and activated keratinocytes in vivo and in vitro. Biochem J. 1995; 309:263–269.
Article26. Simon GA, Maibach HI. The pig as an experimental animal model of percutaneous permeation in man: qualitative and quantitative observations-an overview. Skin Pharmacol Appl Skin Physiol. 2000; 13:229–234.
Article27. Sonis ST, O'Donnell KE, Popat R, Bragdon C, Phelan S, Cocks D, Epstein JB. The relationship between mucosal cyclooxygenase-2 (COX-2) expression and experimental radiation-induced mucositis. Oral Oncol. 2004; 40:170–176.
Article28. Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001; 9:66–76.
Article29. Wang XJ, Lin S, Kang HF, Dai ZJ, Bai MH, Ma XL, Ma XB, Liu MJ, Liu XX, Wang BF. The effect of RHIZOMA COPTIDIS and COPTIS CHINESIS aqueous extract on radiation-induced skin injury in a rat model. BMC Complement Altern Med. 2013; 13:105.30. Yeoh ASJ, Bowen JM, Gibson RJ, Keefe DMK. Nuclear factor κB (NFκB) and cyclooxygenase-2 (Cox-2) expression in the irradiated colorectum is associated with subsequent histopathological changes. Int J Radiat Oncol Biol Phys. 2005; 63:1295–1303.
Article31. Yeoh ASJ, Gibson RJ, Yeoh EEK, Bowen JM, Stringer AM, Giam KA, Keefe DMK. A novel animal model to investigate fractionated radiotherapy-induced alimentary mucositis: the role of apoptosis, p53, nuclear factor-κB, COX-1, and COX-2. Mol Cancer Ther. 2007; 6:2319–2327.
Article32. Zhou H, Ivanov VN, Lien YC, Davidson M, Hei TK. Mitochondrial function and nuclear factor-κB-mediated signaling in radiation-induced bystander effects. Cancer Res. 2008; 68:2233–2240.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- beta-Lapachone suppresses radiation-induced activation of nuclear factor-kappaB
- NF-kappaB Binding Activity and Cyclooxygenase-2 Expression in Persistent betaCCI(4)-Treated Rat Liver Injury
- TNF-alpha-induced Increase of Human beta-defensin-2 Expression in HaCaT Cell Lines
- PMA-induced up-regulation of MMP-9 is regulated by a PKCalpha-NF-kappaB cascade in human lung epithelial cells
- Salicylate Regulates Cyclooxygenase-2 Expression through ERK and Subsequent NF-kappaB Activation in Osteoblasts