Yonsei Med J.
2013 Jul;54(4):865-874. 10.3349/ymj.2013.54.4.865.
The Impact of Cigarette Smoking on the Frequency of and Qualitative Differences in KRAS Mutations in Korean Patients with Lung Adenocarcinoma
- Affiliations
-
- 1Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea. cbc1971@yuhs.ac
- 2Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Thoracic and Cardiovascular Surgery, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Chemistry, College of Science, Yonsei University, Seoul, Korea.
- 5Department of Biochemistry, College of Life Science & Biotechnology, Yonsei University, Seoul, Korea.
- 6JE UK Institute for Cancer Research, Gumi, Korea.
- 7Brain Korea 21 Project for Medical Sciences, Yonsei University College of Medicine, Seoul, Korea.
- 8Institute for Cancer Research, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
- 9Department of Medical Engineering, Yonsei University College of Medicine, Seoul, Korea.
- 10Clinical Trials Centers for Medical Devices, Yonsei University Health System, Seoul, Korea.
- 11Department of Hematology-Oncology, National University Cancer Institute, National University Health System, Singapore.
- 12Memorial Sloan-Kettering Cancer Center and Weill Medical College of Cornell University, New York, NY, USA.
- KMID: 2158219
- DOI: http://doi.org/10.3349/ymj.2013.54.4.865
Abstract
- PURPOSE
This study was designed to determine the relationship of cigarette smoking to the frequency and qualitative differences among KRAS mutations in lung adenocarcinomas from Korean patients.
MATERIALS AND METHODS
Detailed smoking histories were obtained from 200 consecutively enrolled patients with lung adenocarcinoma according to a standard protocol. EGFR (exons 18 to 21) and KRAS (codons 12/13) mutations were determined via direct-sequencing.
RESULTS
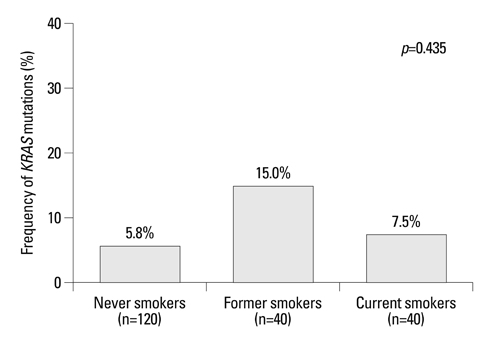
The incidence of KRAS mutations was 8% (16 of 200) in patients with lung adenocarcinoma. KRAS mutations were found in 5.8% (7 of 120) of tumors from never-smokers, 15% (6 of 40) from former-smokers, and 7.5% (3 of 40) from current-smokers. The frequency of KRAS mutations did not differ significantly according to smoking history (p=0.435). Never-smokers were significantly more likely than former or current smokers to have a transition mutation (G-->A or C-->T) rather than a transversion mutation (G-->T or G-->C) that is known to be smoking-related (p=0.011). In a Cox regression model, the adjusted hazard ratios for the risk of progression with epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) were 0.24 (95% CI, 0.14-0.42; p<0.001) for the EGFR mutation and 1.27 (95% CI, 0.58-2.79; p=0.537) for the KRAS mutation.
CONCLUSION
Cigarette smoking did not influence the frequency of KRAS mutations in lung adenocarcinomas in Korean patients, but influenced qualitative differences in the KRAS mutations.
MeSH Terms
-
Adenocarcinoma/drug therapy/etiology/*genetics/pathology
Adult
Aged
Aged, 80 and over
Asian Continental Ancestry Group/genetics
Female
Humans
Incidence
Lung Neoplasms/drug therapy/etiology/*genetics/pathology
Male
Middle Aged
*Mutation
Mutation Rate
Proportional Hazards Models
Proto-Oncogene Proteins/*genetics
Receptor, Epidermal Growth Factor/antagonists & inhibitors/genetics
Smoking/adverse effects/*genetics
Treatment Outcome
ras Proteins/*genetics
Proto-Oncogene Proteins
Receptor, Epidermal Growth Factor
ras Proteins
Figure
Reference
-
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009; 59:225–249.
Article2. Jung M, Kim SH, Hong S, Kang YA, Kim SK, Chang J, et al. Prognostic and predictive value of carcinoembryonic antigen and cytokeratin-19 fragments levels in advanced non-small cell lung cancer patients treated with gefitinib or erlotinib. Yonsei Med J. 2012; 53:931–939.
Article3. Mao C, Qiu LX, Liao RY, Du FB, Ding H, Yang WC, et al. KRAS mutations and resistance to EGFR-TKIs treatment in patients with non-small cell lung cancer: a meta-analysis of 22 studies. Lung Cancer. 2010; 69:272–278.
Article4. Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005; 366:1527–1537.
Article5. Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004; 304:1497–1500.
Article6. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–2139.
Article7. Gandhi J, Zhang J, Xie Y, Soh J, Shigematsu H, Zhang W, et al. Alterations in genes of the EGFR signaling pathway and their relationship to EGFR tyrosine kinase inhibitor sensitivity in lung cancer cell lines. PLoS One. 2009; 4:e4576.
Article8. Jackman DM, Miller VA, Cioffredi LA, Yeap BY, Jänne PA, Riely GJ, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Cancer Res. 2009; 15:5267–5273.
Article9. Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004; 101:13306–13311.
Article10. Douillard JY, Shepherd FA, Hirsh V, Mok T, Socinski MA, Gervais R, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol. 2010; 28:744–752.
Article11. Jackman DM, Yeap BY, Lindeman NI, Fidias P, Rabin MS, Temel J, et al. Phase II clinical trial of chemotherapy-naive patients > or = 70 years of age treated with erlotinib for advanced non-small-cell lung cancer. J Clin Oncol. 2007; 25:760–766.
Article12. Miller VA, Riely GJ, Zakowski MF, Li AR, Patel JD, Heelan RT, et al. Molecular characteristics of bronchioloalveolar carcinoma and adenocarcinoma, bronchioloalveolar carcinoma subtype, predict response to erlotinib. J Clin Oncol. 2008; 26:1472–1478.
Article13. Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005; 2:e17.
Article14. Linardou H, Dahabreh IJ, Kanaloupiti D, Siannis F, Bafaloukos D, Kosmidis P, et al. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol. 2008; 9:962–972.
Article15. van Zandwijk N, Mathy A, Boerrigter L, Ruijter H, Tielen I, de Jong D, et al. EGFR and KRAS mutations as criteria for treatment with tyrosine kinase inhibitors: retro- and prospective observations in non-small-cell lung cancer. Ann Oncol. 2007; 18:99–103.
Article16. Schneider CP, Heigener D, Schott-von-Römer K, Gütz S, Laack E, Digel W, et al. Epidermal growth factor receptor-related tumor markers and clinical outcomes with erlotinib in non-small cell lung cancer: an analysis of patients from german centers in the TRUST study. J Thorac Oncol. 2008; 3:1446–1453.
Article17. Han SW, Kim TY, Hwang PG, Jeong S, Kim J, Choi IS, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2005; 23:2493–2501.
Article18. Riely GJ, Marks J, Pao W. KRAS mutations in non-small cell lung cancer. Proc Am Thorac Soc. 2009; 6:201–205.19. Roberts PJ, Stinchcombe TE, Der CJ, Socinski MA. Personalized medicine in non-small-cell lung cancer: is KRAS a useful marker in selecting patients for epidermal growth factor receptor-targeted therapy? J Clin Oncol. 2010; 28:4769–4777.
Article20. De Luca A, Normanno N. Predictive biomarkers to tyrosine kinase inhibitors for the epidermal growth factor receptor in non-small-cell lung cancer. Curr Drug Targets. 2010; 11:851–864.
Article21. Suda K, Tomizawa K, Mitsudomi T. Biological and clinical significance of KRAS mutations in lung cancer: an oncogenic driver that contrasts with EGFR mutation. Cancer Metastasis Rev. 2010; 29:49–60.
Article22. Ahrendt SA, Decker PA, Alawi EA, Zhu Yr YR, Sanchez-Cespedes M, Yang SC, et al. Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer. 2001; 92:1525–1530.
Article23. Kakegawa S, Shimizu K, Sugano M, Miyamae Y, Kaira K, Araki T, et al. Clinicopathological features of lung adenocarcinoma with KRAS mutations. Cancer. 2011; 117:4257–4266.
Article24. Massarelli E, Varella-Garcia M, Tang X, Xavier AC, Ozburn NC, Liu DD, et al. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin Cancer Res. 2007; 13:2890–2896.
Article25. Han SW, Kim TY, Jeon YK, Hwang PG, Im SA, Lee KH, et al. Optimization of patient selection for gefitinib in non-small cell lung cancer by combined analysis of epidermal growth factor receptor mutation, K-ras mutation, and Akt phosphorylation. Clin Cancer Res. 2006; 12:2538–2544.
Article26. Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008; 372:1809–1818.
Article27. Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005; 40:90–97.
Article28. Riely GJ, Kris MG, Rosenbaum D, Marks J, Li A, Chitale DA, et al. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin Cancer Res. 2008; 14:5731–5734.
Article29. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. European Organization for Research and Treatment of Cancer. National Cancer Institute of the United States. National Cancer Institute of Canada. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000; 92:205–216.
Article30. Li M, Liu L, Liu Z, Yue S, Zhou L, Zhang Q, et al. The status of KRAS mutations in patients with non-small cell lung cancers from mainland China. Oncol Rep. 2009; 22:1013–1020.
Article31. Keohavong P, DeMichele MA, Melacrinos AC, Landreneau RJ, Weyant RJ, Siegfried JM. Detection of K-ras mutations in lung carcinomas: relationship to prognosis. Clin Cancer Res. 1996; 2:411–418.32. Rosell R, Monzó M, Pifarré A, Ariza A, Sánchez JJ, Moreno I, et al. Molecular staging of non-small cell lung cancer according to K-ras genotypes. Clin Cancer Res. 1996; 2:1083–1086.33. Sakuma Y, Matsukuma S, Yoshihara M, Nakamura Y, Noda K, Nakayama H, et al. Distinctive evaluation of nonmucinous and mucinous subtypes of bronchioloalveolar carcinomas in EGFR and K-ras gene-mutation analyses for Japanese lung adenocarcinomas: confirmation of the correlations with histologic subtypes and gene mutations. Am J Clin Pathol. 2007; 128:100–108.
Article34. Bae NC, Chae MH, Lee MH, Kim KM, Lee EB, Kim CH, et al. EGFR, ERBB2, and KRAS mutations in Korean non-small cell lung cancer patients. Cancer Genet Cytogenet. 2007; 173:107–113.
Article35. Wu CC, Hsu HY, Liu HP, Chang JW, Chen YT, Hsieh WY, et al. Reversed mutation rates of KRAS and EGFR genes in adenocarcinoma of the lung in Taiwan and their implications. Cancer. 2008; 113:3199–3208.
Article36. Lee YJ, Kim JH, Kim SK, Ha SJ, Mok TS, Mitsudomi T, et al. Lung cancer in never smokers: change of a mindset in the molecular era. Lung Cancer. 2011; 72:9–15.
Article37. Naumov GN, Nilsson MB, Cascone T, Briggs A, Straume O, Akslen LA, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res. 2009; 15:3484–3494.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- EGFR and KRAS Mutations in Patients With Adenocarcinoma of the Lung
- Associations between the Frequency of Electronic Cigarette Use and Smoking-related Characteristics among Adolescent Smokers
- Gender Differences of Susceptibility to Lung Cancer According to Smoking Habits
- Non-small Cell Lung Cancer with Concomitant EGFR, KRAS, and ALK Mutation: Clinicopathologic Features of 12 Cases
- Detection of EGFR and KRAS Mutation by Pyrosequencing Analysis in Cytologic Samples of Non-Small Cell Lung Cancer