Yonsei Med J.
2005 Oct;46(5):687-692. 10.3349/ymj.2005.46.5.687.
The Involvement of Adult Stem Cells Originated from Bone Marrow in the Pathogenesis of Pterygia
- Affiliations
-
- 1Department of Ophthalmology, College of Medicine, Chung-Ang University, Seoul, Korea. jck50ey@kornet.net
- 2Department of Laboratory Medicine, College of Medicine, Chung-Ang University, Seoul, Korea.
- KMID: 2158150
- DOI: http://doi.org/10.3349/ymj.2005.46.5.687
Abstract
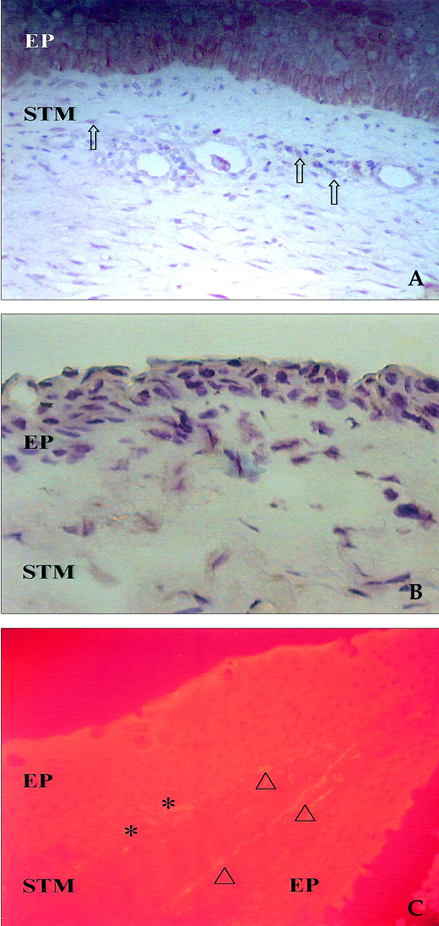
- Pterygium is a proliferative disease. Recent research has reported that stem cells are involved in the pathogenesis of various proliferative diseases, including solid tumors and diabetic proliferate vitreoretinopathy. In previous literature, we hypothesized that adult stem cells originated from bone marrow were involved in the pathogenesis of pterygium. We proved this by immunohistochemical staining with various stem cell markers. The staining showed adult stem cells in the pterygium. c-kit positive cells were observed primarily in the stroma, and some cells were also found in the basal epithelium. AC133 and CD34 positive cells were primarily found in the basal epithelium and were ovoid shaped, similar to the c-kit cells. However, some cells were found in vascular endothelium. STRO-1 positive cells were found mainly in the stroma and were spindle shaped. In recurrent pterygium, cells were more scattered and the expression pattern was denser. Therefore, we suggest a new theory of pterygium pathogenesis. Inflammation caused by environmental factors triggers the abnormal production of some growth factors and cytokines in order to recover from cellular damage. If these healing signals are excessive, limbal basal cells will be changed to abnormally-altered pterygial cells. The excessive wound healing process and remnant altered cells result in recurrence using the same mechanism.
Keyword
MeSH Terms
Figure
Reference
-
1. Holland EJ, Mannis MJ. Ocular surface disease. Medical and surgical management. 2002. New York: Springer-Verlag;65–89.2. Jaros PA, DeLuise VP. Pingueculae and pterygia. Surv Ophthalmol. 1988. 3:41–49.3. Dushku N, John MK, Schultz GS, Reid TW. Pterygia Pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol. 2001. 119:695–706.4. Maini R, Collison DJ, Maidment JM, Davies PD, Wormstone IM. Pterygial derived fibroblasts express functionally active histamine and epidermal growth factor receptors. Exp Eye Res. 2002. 74:237–244.5. Saw SM, Tan D. Pterygium: prevalence, demography and risk factors. Ophthalmic Epidemiol. 1999. 6:219–228.6. Austin P, Jakobiec FA, Iwamoto T. Elastodysplasia and elastodystrophy as the pathologic bases of ocular pterygia and pinguecula. Ophthalmology. 1983. 90:96–109.7. Tan DT, Tang WY, Liu YP, Goh HS, Smith DR. Apoptosis and apoptosis related gene expression in normal conjunctiva and pterygium. Br J Ophthalmol. 2000. 84:212–216.8. Chowers I, Pe'er J, Zamir E, Livni N, Ilsar M, Frucht-Pery J. Proliferative activity and p53 expression in primary and recurrent pterygia. Ophthalmology. 2001. 108:985–988.9. Tan DT, Lim AS, Goh HS, Smith DR. Abnormal expression of the p53 tumor suppressor gene in the conjunctiva of patients with pterygium. Am J Ophthalmol. 1997. 123:404–405.10. Weinstein O, Rosenthal G, Zirkin H, Monos T, Lifshitz T, Argov S. Overexpression of p53 tumor suppressor gene in pterygia. Eye. 2002. 16:619–621.11. Varinli S, Varinli I, Koksal Erkisi M, Doran F. Human papillomavirus in pterygium. Cent Afr J Med. 1994. 40:24–26.12. Dick JE. Stem cells: Self-renewal writ in blood. Nature. 2003. 423:231–233.13. Horwitz EM. Stem cell plasticity: a new image of the bone marrow stem cell. Curr Opin Pediatr. 2003. 15:32–37.14. Ohgushi H, Kitamura S, Kotobuki N, Hirose M, Machida H, Muraki K, et al. Clinical application of marrw mesenchymal stem cells for hard tissue repair. Yonsei Med J. 2004. 45:Suppl. 61–67.15. Nowicki MO, Pawlowski P, Fischer T, Hess G, Pawlowski T, Skorski T. Chronic myelogenous leukemia molecular signature. Oncogene. 2003. 22:3952–3963.16. Pathak S. Organ- and tissue-specific stem cells and carcinogenesis. Anticancer Res. 2002. 22:1353–1356.17. Tu SM, Lin SH, Logothetis CJ. Stem-cell origin of metastasis and heterogeneity in solid tumours. Lancet Oncol. 2002. 3:508–513.18. Risitano AM, Holada K, Chen G, Simak J, Vostal JG, Young NS, et al. CD34+ cells from paroxysmal nocturnal hemoglobinuria (PNH) patients are deficient in surface expression of cellular prion protein (PrPc). Exp Hematol. 2003. 31:65–72.19. Nakakuma H, Kawakuchi T. Pathogenesis of selective expansion of PNH clones. Int J Hematol. 2003. 77:121–124.20. Walshe R, Esser P, Wiedemann P, Heimann K. Proliferative retinal diseases: myofibroblasts cause chronic vitreoretinal traction. Br J Ophthalmol. 1992. 76:550–552.21. Piras F, Moore PS, Ugalde J, Perra MT, Scarpa A, Sirigu P. Detection of human papillomavirus DNA in pterygia from different geographical regions. Br J Ophthalmol. 2003. 87:864–866.22. Detorakis ET, Sourvinos G, Spandidos DA. Detection of herpes simplex virus and human papilloma virus in ophthalmic pterygium. Cornea. 2001. 20:164–167.23. Krause DS, Ito T, Fackler MJ, Smith OM, Collector MI, Sharkis SJ, et al. Characterization of murine CD34, a marker for hematopoietic progenitor and stem cells. Blood. 1994. 84:691–701.24. Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000. 95:952–958.25. Gronthos S, Graves SE, Ohta S, Simmons PJ. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994. 84:4164–4173.26. Dennis JE, Carbillet JP, Caplan AI, Charbord P. The STRO-1+ marrow cell population is multipotential. Cells Tissues Organs. 2002. 170:73–82.27. Lee SB, Li DQ, Tan DT, Meller DC, Tseng SC. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000. 20:325–334.28. Di Girolamo N, Kumar RK, Coroneo MT, Wakefield D. UVB-mediated induction of interleukin-6 and -8 in pterygia and cultured human pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2002. 43:3430–3437.29. Li DQ, Lee SB, Gunja-Smith Z, Liu Y, Solomon A, Meller D, et al. Overexpression of collagenase (MMP-1) and stromelysin (MMP-3) by pterygium head fibroblasts. Arch Ophthalmol. 2001. 119:71–80.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Angiogenic Stem Cells in Bone Regeneration
- Bone marrow-derived stem cells contribute to regeneration of the endometrium
- Differentiation of adult canine bone marrow stem cells into neurons
- Stem Cells for Cardiovascular Disease
- Clinical Trials of Adult Stem Cell Therapy in Patients with Ischemic Stroke