J Korean Med Sci.
2013 Jan;28(1):48-54. 10.3346/jkms.2013.28.1.48.
Serum Cystatin C Is a Major Predictor of Vancomycin Clearance in a Population Pharmacokinetic Analysis of Patients with Normal Serum Creatinine Concentrations
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Bundang Hospital, Seongnam, Korea.
- 2Department of Internal Medicine, Yonsei University College of Medicine and Gangnam Severance Hospital, Seoul, Korea. imfell@yuhs.ac
- KMID: 2158000
- DOI: http://doi.org/10.3346/jkms.2013.28.1.48
Abstract
- We developed a population pharmacokinetic model of vancomycin by integrating the effects of cystatin C and other demographic factors in a large population of Korean patients with normal serum creatinine concentrations to elucidate the precise role of serum cystatin C concentrations in the prediction of vancomycin clearance. A population pharmacokinetic model of vancomycin was developed using NONMEM software from a total of 1,373 vancomycin concentration measurements in 678 patients whose serum creatinine concentrations were lower than 1.2 mg/dL. Covariate selection revealed that cystatin C was the most influential factor and had negative influence (-0.78) in the relationship. Total body weight, sex, age, and serum creatinine were also significantly correlated with the clearance. The estimated intersubject variabilities of clearance and volume of distribution were 24.7% and 25.1%, respectively. A 14-fold difference in predicted trough concentrations was observed according to only cystatin C concentrations in a population of simulated individuals with median demographic characteristics. The use of serum cystatin C as marker of vancomycin clearance for more accurate predictions of serum vancomycin concentrations could be useful, particularly among patients with normal serum creatinine concentrations.
Keyword
MeSH Terms
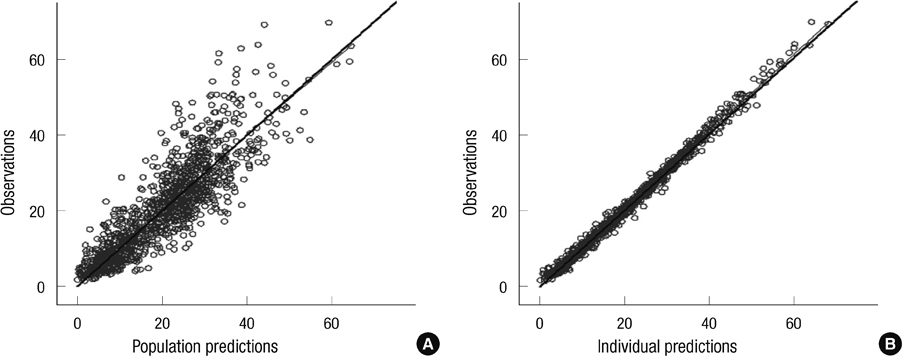
Figure
Cited by 1 articles
-
Underestimation of the Calculated Area Under the Concentration-Time Curve Based on Serum Creatinine for Vancomycin Dosing
Sung Joon Jin, Ji Hyun Yoon, Bo Sook Ahn, Ji Ah Chung, Young Goo Song
Infect Chemother. 2014;46(1):21-29. doi: 10.3947/ic.2014.46.1.21.
Reference
-
1. Appelbaum PC. Microbiology of antibiotic resistance in Staphylococcus aureus. Clin Infect Dis. 2007. 45:Suppl 3. S165–S170.2. Iwamoto T, Kagawa Y, Kojima M. Clinical efficacy of therapeutic drug monitoring in patients receiving vancomycin. Biol Pharm Bull. 2003. 26:876–879.3. MacGowan AP. Pharmacodynamics, pharmacokinetics, and therapeutic drug monitoring of glycopeptides. Ther Drug Monit. 1998. 20:473–477.4. Caregaro L, Menon F, Angeli P, Amodio P, Merkel C, Bortoluzzi A, Alberino F, Gatta A. Limitations of serum creatinine level and creatinine clearance as filtration markers in cirrhosis. Arch Intern Med. 1994. 154:201–205.5. Ross EA, Wilkinson A, Hawkins RA, Danovitch GM. The plasma creatinine concentration is not an accurate reflection of the glomerular filtration rate in stable renal transplant patients receiving cyclosporine. Am J Kidney Dis. 1987. 10:113–117.6. Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002. 40:221–226.7. Filler G, Bokenkamp A, Hofmann W, Le Bricon T, Martinez-Bru C, Grubb A. Cystatin C as a marker of GFR--history, indications, and future research. Clin Biochem. 2005. 38:1–8.8. Hermida J, Tutor JC. Serum cystatin C for the prediction of glomerular filtration rate with regard to the dose adjustment of amikacin, gentamicin, tobramycin, and vancomycin. Ther Drug Monit. 2006. 28:326–331.9. Okamoto G, Sakamoto T, Kimura M, Ukishima Y, Sonoda A, Mori N, Kato Y, Maeda T, Kagawa Y. Serum cystatin C as a better marker of vancomycin clearance than serum creatinine in elderly patients. Clin Biochem. 2007. 40:485–490.10. Suzuki A, Imanishi Y, Nakano S, Niwa T, Ohmori T, Shirai K, Yoshida S, Furuta N, Takemura M, Ito H, et al. Usefulness of serum cystatin C to determine the dose of vancomycin in critically ill patients. J Pharm Pharmacol. 2010. 62:901–907.11. Jin SJ, Bae SC, Kim HW, Kim HK, Na EJ, Ahn BS, Choi JY, Kim CO, Kim JM, Song YG. Evaluation of the effect of initial dose of vancomycin using serum cystatin C as a marker in elderly patients. Infect Chemother. 2009. 41:224–229.12. Tanaka A, Aiba T, Otsuka T, Suemaru K, Nishimiya T, Inoue T, Murase M, Kurosaki Y, Araki H. Population pharmacokinetic analysis of vancomycin using serum cystatin C as a marker of renal function. Antimicrob Agents Chemother. 2010. 54:778–782.13. Jonsson EN, Karlsson MO. Xpose--an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999. 58:51–64.14. Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit--a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005. 79:241–257.15. Parke J, Holford NHG, Charles BG. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Methods Programs Biomed. 1999. 59:19–29.16. Wade JR, Beal SL, Sambol NC. Interaction between structural, statistical, and covariate models in population pharmacokinetic analysis. J Pharmacokinet Biopharm. 1994. 22:165–177.17. Nielsen EI, Sandstrom M, Honore PH, Ewald U, Friberg LE. Developmental pharmacokinetics of gentamicin in preterm and term neonates: population modelling of a prospective study. Clin Pharmacokinet. 2009. 48:253–263.18. Hoek FJ, Kemperman FA, Krediet RT. A comparison between cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant. 2003. 18:2024–2031.19. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976. 16:31–41.20. Martin JH, Norris R, Barras M, Roberts J, Morris R, Doogue M, Jones GR. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Clin Biochem Rev. 2010. 31:21–24.21. Buelga DS, del Mar Fernandez de Gatta M, Herrera EV, Dominguez-Gil A, Garcia MJ. Population pharmacokinetic analysis of vancomycin in patients with hematological malignancies. Antimicrob Agents Chemother. 2005. 49:4934–4941.22. Llopis-Salvia P, Jimenez-Torres NV. Population pharmacokinetic parameters of vancomycin in critically ill patients. J Clin Pharm Ther. 2006. 31:447–454.23. Sanchez JL, Dominguez AR, Lane JR, Anderson PO, Capparelli EV, Cornejo-Bravo JM. Population pharmacokinetics of vancomycin in adult and geriatric patients: comparison of eleven approaches. Int J Clin Pharmacol Ther. 2010. 48:525–533.24. Thomson AH, Staatz CE, Tobin CM, Gall M, Lovering AM. Development and evaluation of vancomycin dosage guidelines designed to achieve new target concentrations. J Antimicrob Chemother. 2009. 63:1050–1057.25. Savic RM, Karlsson MO. Importance of shrinkage in empirical bayes estimates for diagnostics: problems and solutions. AAPS J. 2009. 11:558–569.26. Ducharme MP, Slaughter RL, Edwards DJ. Vancomycin pharmacokinetics in a patient population: effect of age, gender, and body weight. Ther Drug Monit. 1994. 16:513–518.27. Yasuhara M, Iga T, Zenda H, Okumura K, Oguma T, Yano Y, Hori R. Population pharmacokinetics of vancomycin in Japanese adult patients. Ther Drug Monit. 1998. 20:139–148.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the Effect of Initial dose of Vancomycin using Serum Cystatin C as a Marker in Elderly Patients
- A Meta-Analysis on the Performance of Cystatin C- versus Creatinine-based eGFR Equations in Predicting Vancomycin Clearance
- Usefulness of serum cystatin C to determine the dose of vancomycin in neonate
- Serum Cystatin C for the Evaluation of Renal Function in the Spinal Cord Injured Patients
- Relationship between serum cystatin C and glomerular filtration rate in renal transplant patients