J Korean Med Sci.
2011 Mar;26(3):317-324. 10.3346/jkms.2011.26.3.317.
Suppression of CFTR-mediated Cl- Secretion of Airway Epithelium in Vitamin C-deficient Mice
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. jkane.kim@samsung.com
- 2Department of Anatomy, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Physiology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2157866
- DOI: http://doi.org/10.3346/jkms.2011.26.3.317
Abstract
- Hyperoxic ventilation induces detrimental effects on the respiratory system, and ambient oxygen may be harmful unless compensated by physiological anti-oxidants, such as vitamin C. Here we investigate the changes in electrolyte transport of airway epithelium in mice exposed to normobaric hyperoxia and in gulonolacton oxidase knock-out (gulo[-/-]) mice without vitamin C (Vit-C) supplementation. Short-circuit current (Isc) of tracheal epithelium was measured using Ussing chamber technique. After confirming amiloride-sensitive Na+ absorption (DeltaIsc,amil), cAMP-dependent Cl- secretion (DeltaIsc,forsk) was induced by forskolin. To evaluate Ca2+-dependent Cl- secretion, ATP was applied to the luminal side (DeltaIsc,ATP). In mice exposed to 98% PO2 for 36 hr, DeltaIsc,forsk decreased, DeltaIsc,amil and DeltaIsc,ATP was not affected. In gulo(-/-) mice, both DeltaIsc,forsk and DeltaIsc,ATP decreased from three weeks after Vit-C deprivation, while both were unchanged with Vit-C supplementation. At the fourth week, tissue resistance and all electrolyte transport activities were decreased. An immunofluorescence study showed that the expression of cystic fibrosis conductance regulator (CFTR) was decreased in gulo(-/-) mice, whereas the expression of KCNQ1 K+ channel was preserved. Taken together, the CFTR-mediated Cl- secretion of airway epithelium is susceptible to oxidative stress, which suggests that supplementation of the antioxidant might be beneficial for the maintenance of airway surface liquid.
Keyword
MeSH Terms
-
Animals
Ascorbic Acid Deficiency/*metabolism
Biological Transport/drug effects
Chlorides/*metabolism
Cystic Fibrosis Transmembrane Conductance Regulator/antagonists & inhibitors/drug
Forskolin/pharmacology
Hyperbaric Oxygenation
Hyperoxia/*physiopathology
Ion Transport/drug effects
Mice
Mice, Inbred C57BL
Mice, Inbred ICR
Mice, Knockout/metabolism
Mice, Transgenic
Microscopy, Fluorescence
Oxidative Stress
Oxygen/adverse effects/pharmacology
Potassium Channels/metabolism
Respiratory Mucosa/drug effects/*metabolism/secretion
Sodium
Sugar Acids/metabolism
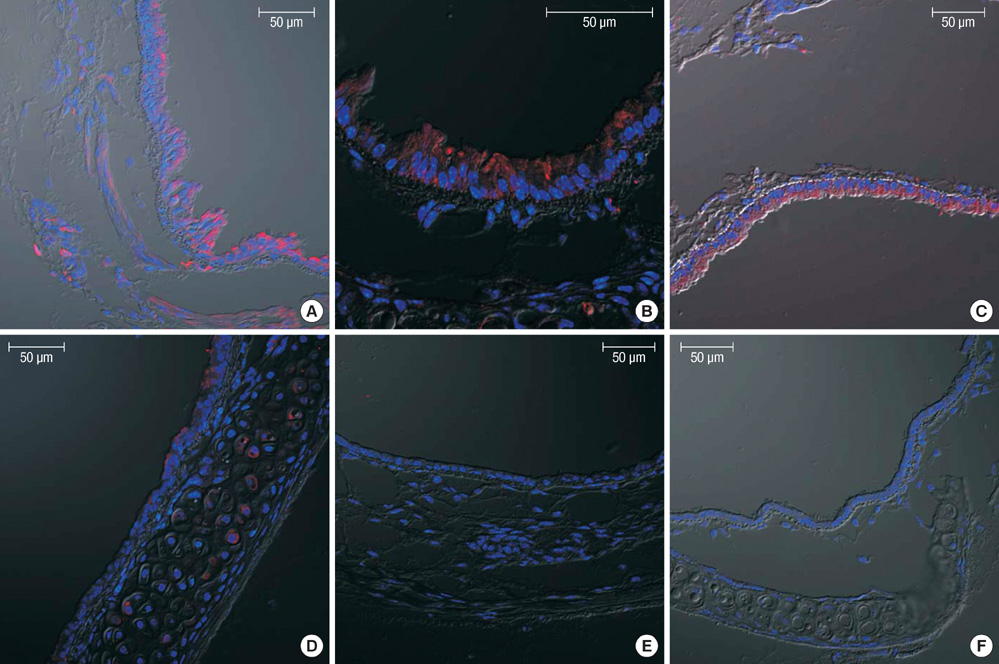
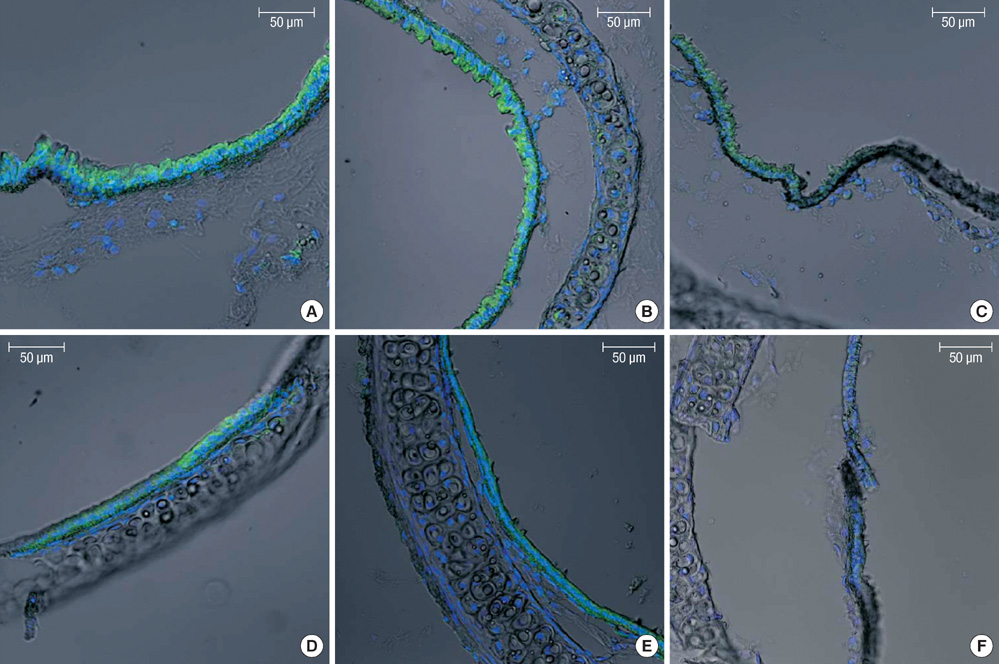
Figure
Reference
-
1. Saugstad OD. Chronic lung disease: oxygen dogma revisited. Acta Paediatr. 2001. 90:113–115.2. Sola A. Oxygen in neonatal anesthesia: friend or foe? Curr Opin Anaesthesiol. 2008. 21:332–339.3. Brozmanova M, Plevkova J, Bartos V, Plank L, Javorka M, Tatar M. The interaction of dietary antioxidant vitamins and oxidative stress on cough reflex in guinea-pigs after long term oxygen therapy. J Physiol Pharmacol. 2006. 57:Suppl 4. 45–54.4. Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. 2007. 4th ed. Oxford: Oxford University Press;1–29.5. Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006. 173:1139–1144.6. Cantin AM, Bilodeau G, Ouellet C, Liao J, Hanrahan JW. Oxidant stress suppresses CFTR expression. Am J Physiol Cell Physiol. 2006. 290:C262–C270.7. Jeulin C, Guadagnini R, Marano F. Oxidant stress stimulates Ca2+-activated chloride channels in the apical activated membrane of cultured nonciliated human nasal epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005. 289:L636–L646.8. Mohsenin V. Effect of vitamin C on NO2-induced airway hyperresponsiveness in normal subjects. A randomized double-blind experiment. Am Rev Respir Dis. 1987. 136:1408–1411.9. Kelly FJ, Mudway I, Blomberg A, Frew A, Sandström T. Altered lung antioxidant status in patients with mild asthma. Lancet. 1999. 354:482–483.10. van der Vliet A, O'Neill CA, Cross CE, Koostra JM, Volz WG, Halliwell B, Louie S. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am J Physiol. 1999. 276:L289–L296.11. Chambers LA, Rollins BM, Tarran R. Liquid movement across the surface epithelium of large airways. Respir Physiol Neurobiol. 2007. 159:256–270.12. Fischer H, Schwarzer C, Illek B. Vitamin C controls the cystic fibrosis transmembrane conductance regulator chloride channel. Proc Natl Acad Sci U S A. 2004. 101:3691–3696.13. Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004. 23:146–158.14. Cowley EA, Linsdell P. Oxidant stress stimulates anion secretion from the human airway epithelial cell line Calu-3: implications for cystic fibrosis lung disease. J Physiol. 2002. 543:201–209.15. Schwarzer C, Fischer H, Kim EJ, Barber KJ, Mills AD, Kurth MJ, Gruenert DC, Suh JH, Machen TE, Illek B. Oxidative stress caused by pyocyanin impairs CFTR Cl(-) transport in human bronchial epithelial cells. Free Radic Biol Med. 2008. 45:1653–1662.16. Maeda N, Hagihara H, Nakata Y, Hiller S, Wilder J, Reddick R. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci USA. 2000. 97:841–846.17. Cotton CU. Basolateral potassium channels and epithelial ion transport. Am J Respir Cell Mol Biol. 2000. 23:270–272.18. Grahammer F, Warth R, Barhanin J, Bleich M, Hug MJ. The small conductance K+ channel, KCNQ1: expression, function, and subunit composition in murine trachea. J Biol Chem. 2001. 276:42268–42275.19. Schreiber R, Kunzelmann K. Purinergic P2Y6 receptors Induce Ca2+ and CFTR dependent Cl- secretion in mouse trachea. Cell Physiol Biochem. 2005. 16:99–108.20. Paradiso AM, Ribeiro CM, Boucher RC. Polarized signaling via purinoceptors in normal and cystic fibrosis airway epithelia. J Gen Physiol. 2001. 117:53–67.21. Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J. 2000. 16:534–554.22. van der Vliet A, Cross CE. Oxidants, nitrosants, and the lung. Am J Med. 2000. 109:398–421.23. Dworski R. Oxidant stress in asthma. Thorax. 2000. 55:Suppl 2. S51–S53.24. Lamb NJ, Gutteridge JM, Baker C, Evans TW, Quinlan GJ. Oxidative damage to proteins of bronchoalveolar lavage fluid in patients with acute respiratory distress syndrome: evidence for neutrophil-mediated hydroxylation, nitration, and chlorination. Crit Care Med. 1999. 27:1738–1744.25. Powers WF, Clemens J. Risk factors for bronchopulmonary dysplasia. J Pediatr. 1992. 120:667–668.26. Cantin AM, White TB, Cross CE, Forman HJ, Sokol RJ, Borowitz D. Antioxidants in cystic fibrosis. Conclusions from the CF antioxidant workshop, Bethesda, Maryland, November 11-12, 2003. Free Radic Biol Med. 2007. 42:15–31.27. Bebok Z, Varga K, Hicks JK, Venglarik CJ, Kovacs T, Chen L, Hardiman KM, Collawn JF, Sorscher EJ, Matalon S. Reactive oxygen nitrogen species decrease cystic fibrosis transmembrane conductance regulator expression and cAMP-mediated Cl- secretion in airway epithelia. J Biol Chem. 2002. 277:43041–43049.28. Rhee JE, Jung SE, Shin SD, Suh GJ, Noh DY, Youn YK, Oh SK, Choe KJ. The effects of antioxidants and nitric oxide modulators on hepatic ischemic-reperfusion injury in rats. J Korean Med Sci. 2002. 17:502–506.29. Lee GJ, Chung HW, Lee KH, Ahn HS. Antioxidant vitamins and lipid peroxidation in patients with cervical intraepithelial neoplasia. J Korean Med Sci. 2005. 20:267–272.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Uridine-5'-Triphosphate Stimulates Chloride Secretion via Cystic Fibrosis Transmembrane Conductance Regulator and Ca2+-Activated Chloride Channels in Cultured Human Middle Ear Epithelial Cells
- Anion interactions with CFTR and consequences for HCO3- transport in secretory epithelia
- Localization of Cystic Fibrosis Transmembrane Conductance Regulator in Nasal Polyp Epithelial Cell
- Relation between the Cystic Fibrosis Transmembrane Conductance Regulators ( CFTR ) in Biliary Epithelium and Development of Intrahepatic Choledocholithiasis
- Variation of pH and Electrolyte in Nasal Secretum