J Korean Med Sci.
2010 Oct;25(10):1418-1426. 10.3346/jkms.2010.25.10.1418.
Stemness Evaluation of Mesenchymal Stem Cells from Placentas According to Developmental Stage: Comparison to Those from Adult Bone Marrow
- Affiliations
-
- 1Institute of Stem Cell Research, Korea University, Seoul, Korea. kbs0309@korea.ac.kr
- 2Department of Obstetrics and Gynecology, Korea University Medical Center, Seoul, Korea.
- 3Department of Internal Medicine, Korea University Medical Center, Seoul, Korea.
- KMID: 2157843
- DOI: http://doi.org/10.3346/jkms.2010.25.10.1418
Abstract
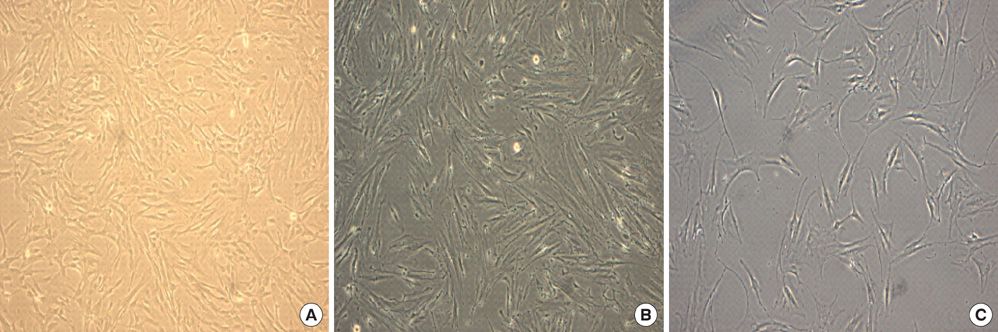
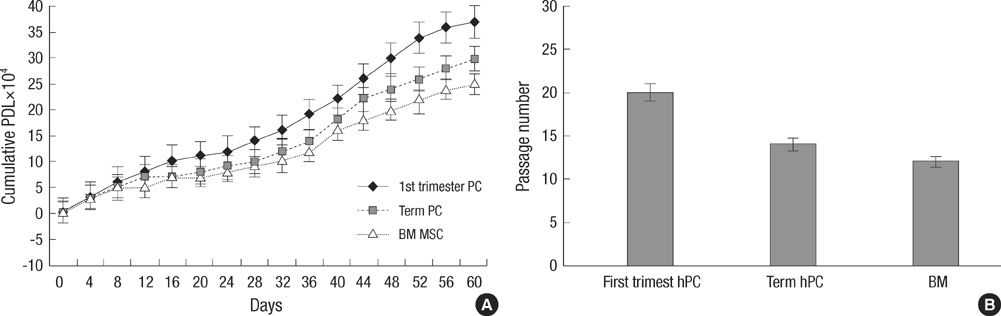
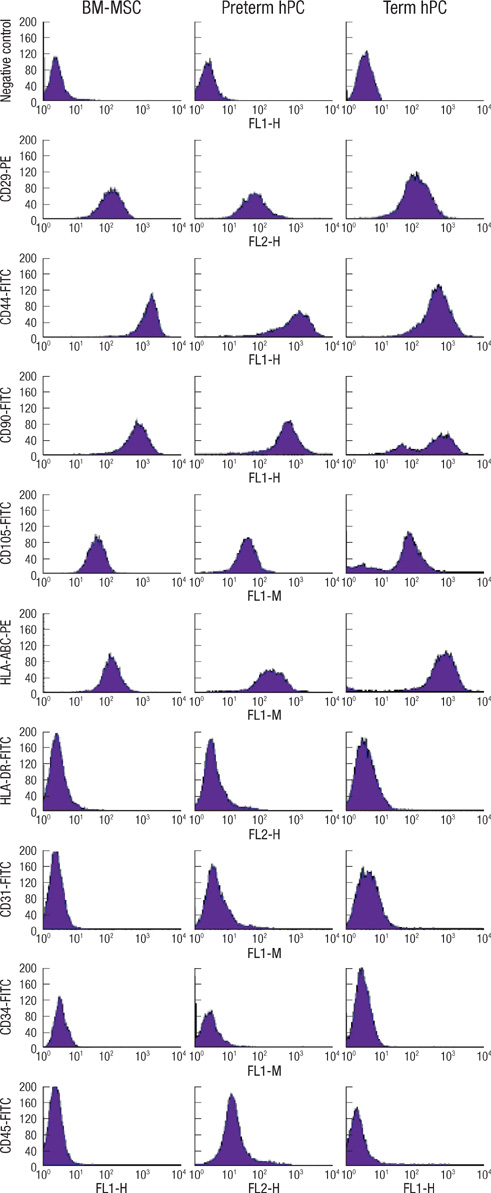
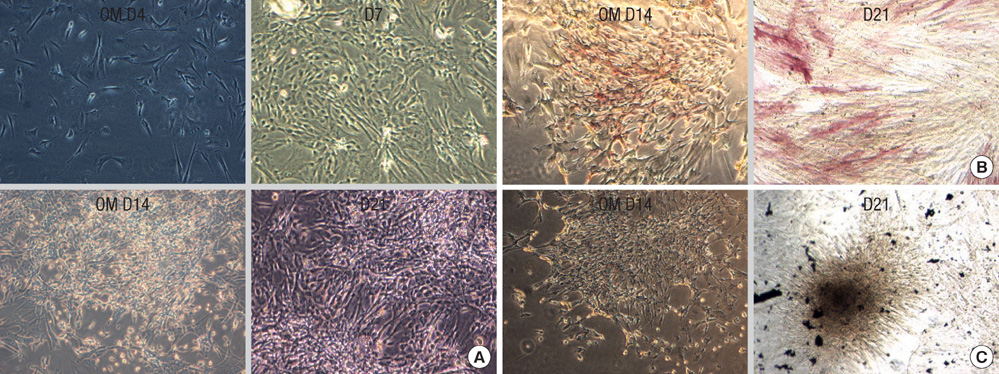
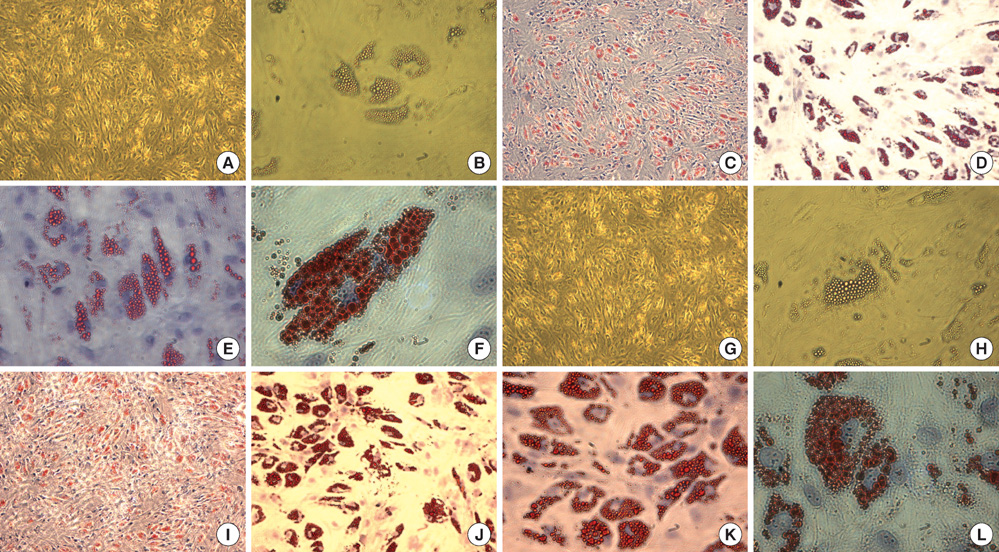
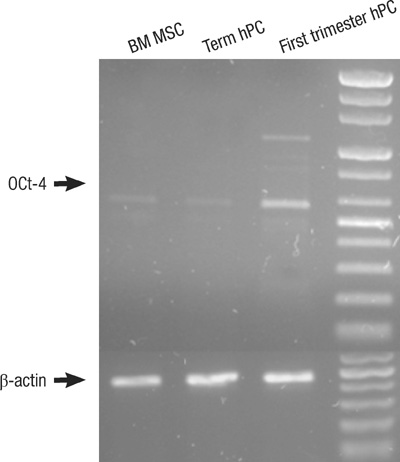
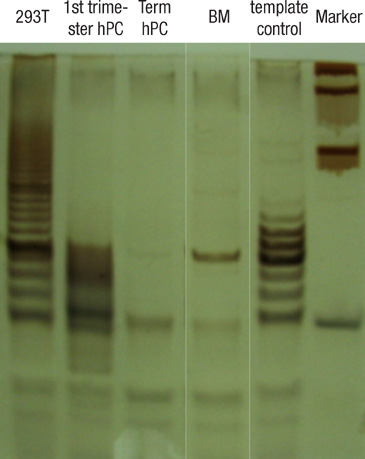
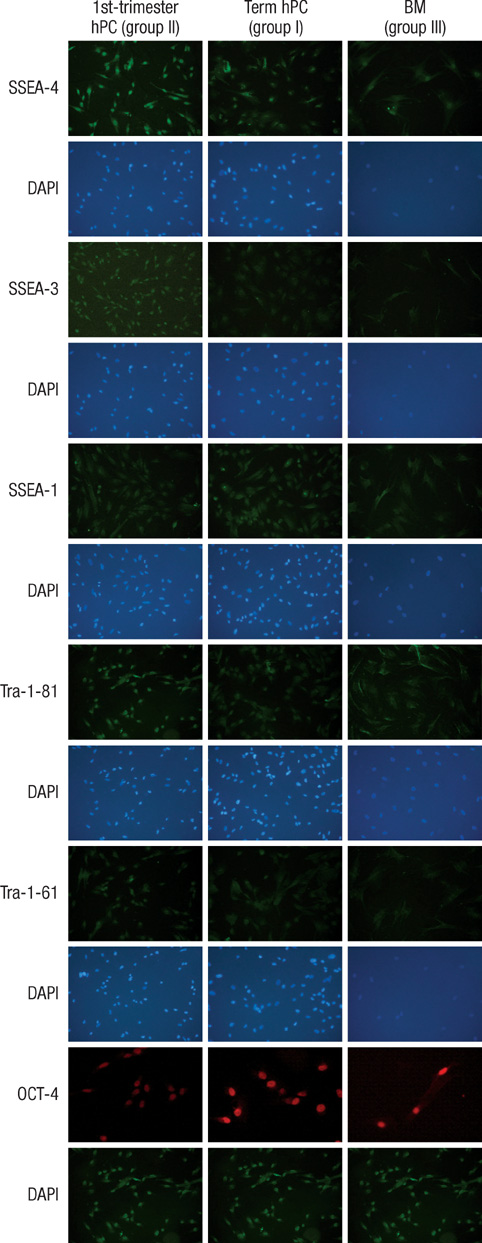
- This study was done to evaluate the stemness of human mesenchymal stem cells (hMSCs) derived from placenta according to the development stage and to compare the results to those from adult bone marrow (BM). Based on the source of hMSCs, three groups were defined: group I included term placentas, group II included first-trimester placentas, and group III included adult BM samples. The stemness was evaluated by the proliferation capacity, immunophenotypic expression, mesoderm differentiation, expression of pluripotency markers including telomerase activity. The cumulative population doubling, indicating the proliferation capacity, was significantly higher in group II (P<0.001, 31.7+/-5.8 vs. 15.7+/-6.2 with group I, 9.2+/-4.9 with group III). The pattern of immunophenotypic expression and mesoderm differentiation into adipocytes and osteocytes were similar in all three groups. The expression of pluripotency markers including ALP, SSEA-4, TRA-1-60, TRA-1-81, Oct-4, and telomerase were strongly positive in group II, but very faint positive in the other groups. In conclusions, hMSCs from placentas have different characteristics according to their developmental stage and express mesenchymal stemness potentials similar to those from adult human BMs.
Keyword
MeSH Terms
-
Antigens, Surface/metabolism
Bone Marrow Cells/*cytology/metabolism
Cell Proliferation
Female
Humans
Immunophenotyping
Mesenchymal Stem Cells/*cytology/metabolism
Mesoderm/cytology
Octamer Transcription Factor-3/metabolism
Placenta/*cytology/growth & development
Pregnancy
Pregnancy Trimester, First
Proteoglycans/metabolism
Stage-Specific Embryonic Antigens/metabolism
Telomerase/metabolism
Figure
Reference
-
1. Bianco P, Gehron RP. Marrow stromal stem cells. J Clin Invest. 2000. 105:1663–1668.
Article2. Vaanaenaen HK. Mesenchymal stem cells. Ann Med. 2005. 37:469–479.3. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000. 109:235–242.
Article4. Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001. 98:2396–2402.
Article5. Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006. 7:14.6. Barker JN, Davies SM, DeFor T, Ramsay Norma KC, Weisdorf DJ, Wagner JE. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: results of a matched-pair analysis. Blood. 2001. 97:2957–2961.
Article7. Yu M, Xiao Z, Shen L, Li L. Mid-trimester fetal blood-derived adherent cells share characteristics similar to mesenchymal stem cells but full-term umbilical cord blood does not. Br J Haematol. 2004. 124:666–675.
Article8. Haigh T, Chen C, Jones CJ, Aplin JD. Studies of mesenchymal cells from 1st trimester human placenta: expression of cytokeratin outside the trophoblast lineage. Placenta. 1999. 20:615–625.
Article9. Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004. 22:649–658.
Article10. Miao Z, Jin J, Chen L, Zhu J, Huang W, Zhao J, Qian H, Zhang X. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006. 30:681–687.
Article11. Zhang X, Mitsuru A, Igura K, Takahashi K, Ichinose S, Yamaguchi S, Takahashi TA. Mesenchymal progenitor cells derived from chorionic villi of human placenta for cartilage tissue engineering. Biochem Biophys Res Commun. 2006. 340:944–952.
Article12. In't Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004. 22:1338–1345.13. Guillot PV, Gotherstrom C, Chan J, Kurata H, Fisk NM. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells. 2007. 25:646–654.
Article14. Hwang JH, Shim SS, Seok OS, Lee HY, Woo SK, Kim BH, Song HR, Lee JK, Park YK. Comparison of cytokine expression in mesenchymal stem cells from human placenta, cord blood and bone marrow. J Korean Med Sci. 2009. 24:547–554.
Article15. Mikkola HK, Gekas C, Orkin SH, Dieterlen-Lievre F. Placenta as a site for hematopoietic stem cell development. Exp Hematol. 2005. 33:1048–1054.
Article16. Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006. 99:1285–1297.
Article17. Kim SJ, Song CH, Sung HJ, Yoo YD, Geum DH, Park SH, Yoo JH, Oh JH, Shin HJ, Kim SH, Kim JS, Kim BS. Human placenta-derived feeders support prolonged undifferentiated propagation of a human embryonic stem cell line, SNUhES3: comparison with human bone marrow-derived feeders. Stem Cells Dev. 2007. 16:421–428.
Article18. Lee CC, Ye F, Tarantal AF. Comparison of growth and differentiation of fetal and adult rhesus monkey. Stem Cells Dev. 2006. 15:209–220.19. Battula VL, Bareiss PM, Treml S, Conrad S, Albert I, Hojak S, Abele H, Schewe B, Just L, Skutella T, Buhring HJ. Human placenta and bone marrow derived MSC cultured in serum-free, b-FGF-containing medium express cell surface frizzled-9 and SSEA-4 and give rise to multilineage differentiation. Differentiation. 2007. 75:279–291.
Article20. Bernardo ME, Emons JA, Karperien M, Nauta AJ, Willemze R, Roelofs H, Romeo S, Marchini A, Rappold GA, Vukicevic S, Locatelli F, Fibbe WE. Human mesenchymal stem cells derived from bone marrow display a better chondrogenic differentiation compared with other sources. Connect Tissue Res. 2007. 48:132–140.
Article21. Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, Seo BM, Sonoyama W, Zheng JJ, Baker CC, Chen W, Ried T, Shi S. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006. 24:1095–1103.
Article22. Takeuchi M, Takeuchi K, Kohara A, Satoh M, Shioda S, Ozawa Y, Ohtani A, Morita K, Hirano T, Terai M, Umezawa A, Mizusawa H. Chromosomal instability in human mesenchymal stem cells immortalized with human papilloma virus E6, E7, and hTERT genes. In Vitro Cell Dev Biol Anim. 2007. 43:129–138.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative characteristic study from bone marrow-derived mesenchymal stem cells
- Clinical Use of Mesenchymal Stem Cells in Bone Regeneration
- Stemness Signature of Equine Marrow-derived Mesenchymal Stem Cells
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Stem Cells for Cardiovascular Disease