Ann Dermatol.
2011 May;23(2):138-143. 10.5021/ad.2011.23.2.138.
Sox9 Increases the Proliferation and Colony-forming Activity of Outer Root Sheath Cells Cultured In Vitro
- Affiliations
-
- 1Department of Dermatology and Research Institute for Medical Sciences, School of Medicine, Chungnam National University, Daejeon, Korea. cdkimd@cnu.ac.kr
- 2Department of Nursing, Kongju National University, Gongju, Korea.
- KMID: 2156653
- DOI: http://doi.org/10.5021/ad.2011.23.2.138
Abstract
- BACKGROUND
beta-catenin plays a pivotal role in hair follicle development and hair growth cycle.
OBJECTIVE
The aim of this study was to identify beta-catenin-regulated genes in cultured human hair outer root sheath (ORS) cells.
METHODS
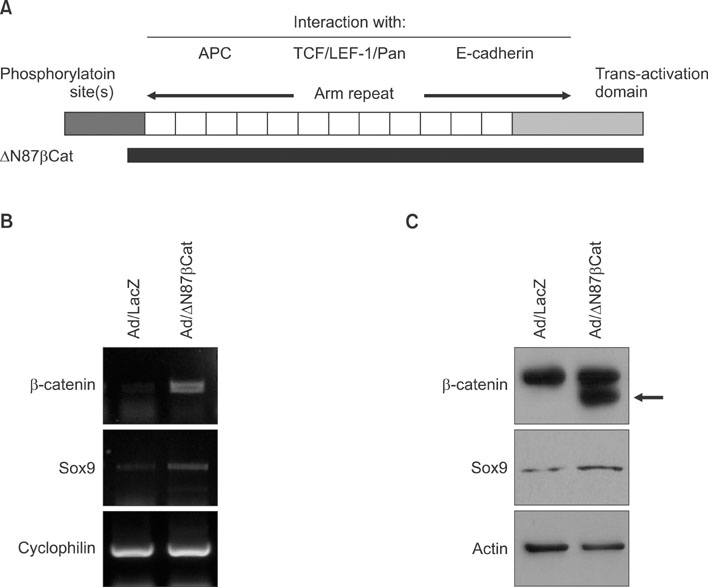
Primary cultured ORS cells were transduced with recombinant adenovirus expressing N-terminal truncated beta-catenin (constitutive active form), and beta-catenin-regulated genes were identified.
RESULTS
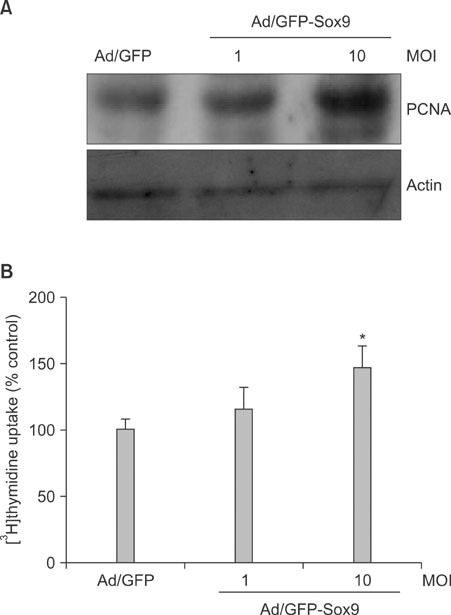
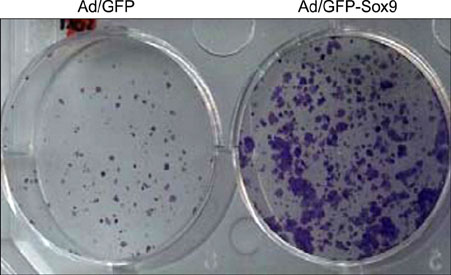
Overexpression of the constitutively active form of beta-catenin led to induction of Sox9 expression at both mRNA and protein levels. To investigate the potential role of Sox9, we made the recombinant adenovirus expressing green fluorescent protein-tagged Sox9, and then transduced into cultured ORS cells. Interestingly, Sox9 induced the expression of keratin 15, increased the proliferation of ORS cells in vitro, and enhanced colony-forming activity.
CONCLUSION
Our results suggest that Sox9 is a beta-catenin-regulated gene in ORS cells, and has potential importance in the regulation of hair follicle homeostasis.
Keyword
MeSH Terms
Figure
Reference
-
1. Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999. 341:491–497.
Article2. Hülsken J, Behrens J. The Wnt signalling pathway. J Cell Sci. 2000. 113:3545.
Article3. Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell. 1998. 95:605–614.
Article4. Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001. 105:533–545.
Article5. Sohn KC, Shi G, Jang S, Choi DK, Lee Y, Yoon TJ, et al. Pitx2, a β-catenin-regulated transcription factor, regulates the differentiation of outer root sheath cells cultured in vitro. J Dermatol Sci. 2009. 54:6–11.
Article6. Kim JH, Choi DK, Lee SS, Choi SJ, Kim CD, Yoon TJ, et al. Enhancement of keratinocyte differentiation by rose absolute oil. Ann Dermatol. 2010. 22:255–261.
Article7. Wong MH, Rubinfeld B, Gordon JI. Effects of forced expression of an NH2-terminal truncated β-catenin on mouse intestinal epithelial homeostasis. J Cell Biol. 1998. 141:765–777.
Article8. Vidal VP, Chaboissier MC, Lützkendorf S, Cotsarelis G, Mill P, Hui CC, et al. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol. 2005. 15:1340–1351.
Article9. Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, et al. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006. 116:249–260.
Article10. Yang JS, Lavker RM, Sun TT. Upper human hair follicle contains a subpopulation of keratinocytes with superior in vitro proliferative potential. J Invest Dermatol. 1993. 101:652–659.
Article11. Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc Natl Acad Sci U S A. 2005. 102:14677–14682.
Article12. Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990. 61:1329–1337.
Article13. Van Mater D, Kolligs FT, Dlugosz AA, Fearon ER. Transient activation of β-catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 2003. 17:1219–1224.
Article14. Barrionuevo F, Scherer G. SOX E genes: SOX9 and SOX8 in mammalian testis development. Int J Biochem Cell Biol. 2010. 42:433–436.
Article15. Yano F, Kugimiya F, Ohba S, Ikeda T, Chikuda H, Ogasawara T, et al. The canonical Wnt signaling pathway promotes chondrocyte differentiation in a Sox9-dependent manner. Biochem Biophys Res Commun. 2005. 333:1300–1308.
Article16. Blache P, van de, Duluc I, Domon C, Berta P, Freund JN, et al. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol. 2004. 166:37–47.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Growth Effect of Minoxidil and Minoxidil Sulfate on Cultured Human Keratinocytes and Outer Root Sheath Cells
- Expression of p63 and its association with cell proliferation at different stages of murine hair follicle cycle
- Dieckol Inhibits the Effects of Particulate Matter 10 on Sebocytes, Outer Root Sheath Cells, and Cutibacterium Acnes−Pretreated Mice
- Effects of Black Ginseng Water Extract under the Inflammatory Conditions of Cultured Sebocytes and Outer Root Sheath Cells
- Effects of <10-μm Particulate Matter on Cultured Human Sebocytes and Outer Root Sheath Cells and Usefulness of Siegesbeckia Herba Extract