Hip Pelvis.
2015 Mar;27(1):9-16. 10.5371/hp.2015.27.1.9.
Nonsurgical Treatment Strategies after Osteoporotic Hip Fractures
- Affiliations
-
- 1Department of Orthopaedic Surgery, Keimyung University Dongsan Medical Center, Daegu, Korea. min@dsmc.or.kr
- KMID: 2156014
- DOI: http://doi.org/10.5371/hp.2015.27.1.9
Abstract
- Osteoporosis is a metabolic disease that is increasing in prevalence as people live longer. Because the orthopedic surgeon is frequently the first and often the only physician to manage patients with osteoporotic hip fractures, every effort should be made to prevent future fractures. A multidisciplinary approach is essential in treatment of osteoporotic fractures. Basic treatment includes calcium and vitamin D supplementation, fall prevention, hip protection, and balance and exercise programs. Currently available pharmacologic agents are divided into antiresorptive and anabolic groups. Antiresorptive agents such as bisphosphonates limit bone resorption through inhibition of osteoclastic activity. Anabolic agents such as parathyroid hormone promote bone formation.
Keyword
MeSH Terms
-
Anabolic Agents
Bone Density Conservation Agents
Bone Resorption
Calcium
Diphosphonates
Hip
Hip Fractures*
Humans
Metabolic Diseases
Orthopedics
Osteoclasts
Osteogenesis
Osteoporosis
Osteoporotic Fractures
Parathyroid Hormone
Prevalence
Vitamin D
Anabolic Agents
Bone Density Conservation Agents
Calcium
Diphosphonates
Parathyroid Hormone
Vitamin D
Figure
Cited by 1 articles
-
Clinical Results of Complex Subtrochanteric Femoral Fractures with Long Cephalomedullary Hip Nail
Kwang-kyoun Kim, Yougun Won, Danica H. Smith, Gi-Soo Lee, Hee Young Lee
Hip Pelvis. 2017;29(2):113-119. doi: 10.5371/hp.2017.29.2.113.
Reference
-
1. Shea B, Wells G, Cranney A, et al. Osteoporosis Methodology Group and The Osteoporosis Research Advisory Group. Meta-analyses of therapies for postmenopausal osteoporosis VII Meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosis. Endocr Rev. 2002; 23:552–559.
Article2. Warensjö E, Byberg L, Melhus H, et al. Dietary calcium intake and risk of fracture and osteoporosis: prospective longitudinal cohort study. BMJ. 2011; 342:d1473.
Article3. Bolland MJ, Avenell A, Baron JA, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010; 341:c3691.
Article4. Bolland MJ, Barber PA, Doughty RN, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ. 2008; 336:262–266.
Article5. Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women's Health Initiative limited access dataset and meta-analysis. BMJ. 2011; 342:d2040.
Article6. Lewis JR, Calver J, Zhu K, Flicker L, Prince RL. Calcium supplementation and the risks of atherosclerotic vascular disease in older women: results of a 5-year RCT and a 4.5-year follow-up. J Bone Miner Res. 2011; 26:35–41.
Article7. Paik JM, Curhan GC, Sun Q, et al. Calcium supplement intake and risk of cardiovascular disease in women. Osteoporos Int. 2014; 25:2047–2056.
Article8. Papaioannou A, Morin S, Cheung AM, et al. Scientific Advisory Council of Osteoporosis Canada. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ. 2010; 182:1864–1873.
Article9. Karlsson MK, Gerdhem P, Ahlborg HG. The prevention of osteoporotic fractures. J Bone Joint Surg Br. 2005; 87:1320–1327.
Article10. Black DM, Cummings SR, Karpf DB, et al. Fracture Intervention Trial Research Group. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996; 348:1535–1541.
Article11. Bonnick SL, Beck TJ, Cosman F, Hochberg MC, Wang H, de Papp AE. DXA-based hip structural analysis of onceweekly bisphosphonate-treated postmenopausal women with low bone mass. Osteoporos Int. 2009; 20:911–921.
Article12. Lee YK, Nho JH, Ha YC, Koo KH. Persistence with intravenous zoledronate in elderly patients with osteoporosis. Osteoporos Int. 2012; 23:2329–2333.
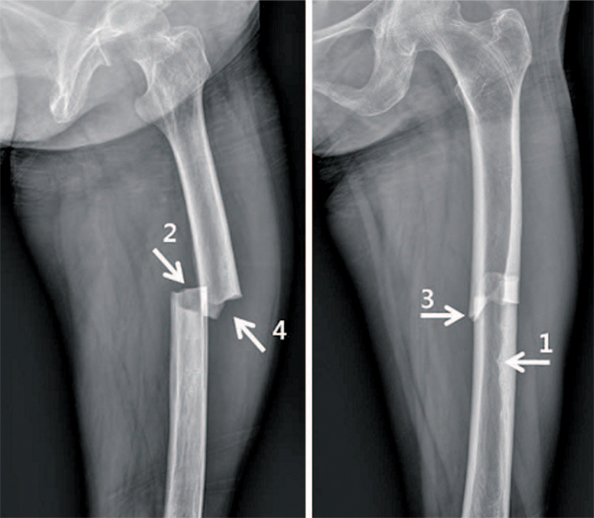
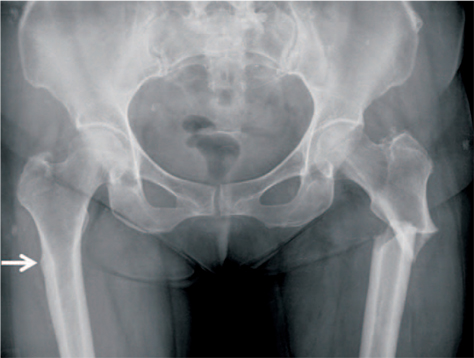
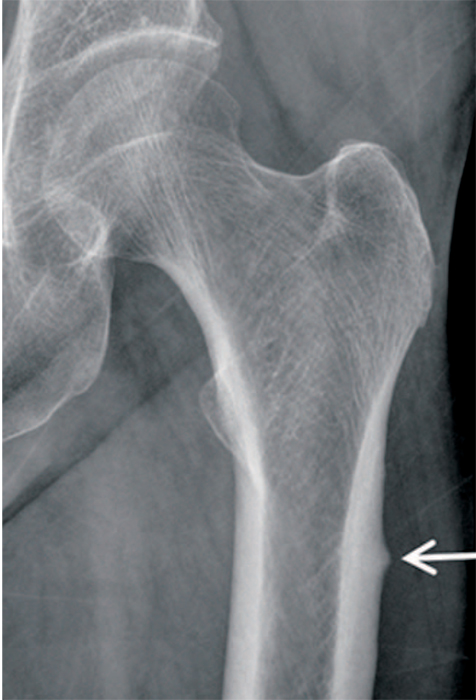
Article13. Carvalho NN, Voss LA, Almeida MO, Salgado CL, Bandeira F. Atypical femoral fractures during prolonged use of bisphosphonates: short-term responses to strontium ranelate and teriparatide. J Clin Endocrinol Metab. 2011; 96:2675–2680.
Article14. Chang JI, Hazboun RC, Chang TI. Incongruities in the AAOMS position paper: medication-related osteonecrosis of the jaw--2014 update. J Oral Maxillofac Surg. 2014; 72:2381.
Article15. Fleisch H. Can bisphosphonates be given to patients with fractures? J Bone Miner Res. 2001; 16:437–440.
Article16. Kim TY, Ha YC, Kang BJ, Lee YK, Koo KH. Does early administration of bisphosphonate affect fracture healing in patients with intertrochanteric fractures? J Bone Joint Surg Br. 2012; 94:956–960.
Article17. Li YT, Cai HF, Zhang ZL. Timing of the initiation of bisphosphonates after surgery for fracture healing: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int. 2015; 26:431–441.
Article18. Komatsubara S, Mori S, Mashiba T, et al. Long-term treatment of incadronate disodium accumulates microdamage but improves the trabecular bone microarchitecture in dog vertebra. J Bone Miner Res. 2003; 18:512–520.
Article19. Komatsubara S, Mori S, Mashiba T, et al. Suppressed bone turnover by long-term bisphosphonate treatment accumulates microdamage but maintains intrinsic material properties in cortical bone of dog rib. J Bone Miner Res. 2004; 19:999–1005.
Article20. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005; 90:1294–1301.
Article21. Lenart BA, Neviaser AS, Lyman S, et al. Association of lowenergy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int. 2009; 20:1353–1362.
Article22. Shane E, Burr D, Ebeling PR, et al. American Society for Bone and Mineral Research. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010; 25:2267–2294.
Article23. Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014; 29:1–23.
Article24. Das De S, Setiobudi T, Shen L, Das De S. A rational approach to management of alendronate-related subtrochanteric fractures. J Bone Joint Surg Br. 2010; 92:679–686.
Article25. Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after longterm bisphosphonate therapy? Clin Orthop Relat Res. 2010; 468:3393–3398.
Article26. Manson JE, Hsia J, Johnson KC, et al. Women's Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003; 349:523–534.
Article27. Ettinger B, Black DM, Mitlak BH, et al. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999; 282:637–645.
Article28. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res Treat. 2001; 65:125–134.
Article29. Miller PD, Chines AA, Christiansen C, et al. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res. 2008; 23:525–535.
Article30. Black DM, Greenspan SL, Ensrud KE, et al. PaTH Study Investigators. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003; 349:1207–1215.
Article31. Ito M, Oishi R, Fukunaga M, et al. The effects of once-weekly teriparatide on hip structure and biomechanical properties assessed by CT. Osteoporos Int. 2014; 25:1163–1172.
Article32. Kawada T. Once-weekly teriparatide administration for 24 weeks in postmenopausal women with osteoporosis. Osteoporos Int. 2014; 25:2321.
Article33. Meunier PJ, Slosman DO, Delmas PD, et al. Strontium ranelate: dose-dependent effects in established postmenopausal vertebral osteoporosis--a 2-year randomized placebo controlled trial. J Clin Endocrinol Metab. 2002; 87:2060–2066.
Article34. Meunier PJ, Roux C, Seeman E, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004; 350:459–468.
Article35. Bolland MJ, Grey A. A comparison of adverse event and fracture efficacy data for strontium ranelate in regulatory documents and the publication record. BMJ Open. 2014; 4:e005787.
Article36. Cummings SR, San Martin J, McClung MR, et al. FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009; 361:756–765.
Article37. Miller PD, Bolognese MA, Lewiecki EM, et al. Amg Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008; 43:222–229.
Article38. Yasuda Y, Kaleta J, Brömme D. The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics. Adv Drug Deliv Rev. 2005; 57:973–993.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Osteoporotic Hip Fracture: How We Make Better Results?
- Clinical Efficacy of Korean FRAX(R) Model in Patients with Hip Fracture
- Surgical or nonsurgical treatment of osteoporotic fractures
- Effect of Sarcopenia on Postoperative Mortality in Osteoporotic Hip Fracture Patients
- Disability Weights for Osteoporosis and Osteoporotic Fractures in South Korea