Neurointervention.
2016 Mar;11(1):30-36. 10.5469/neuroint.2016.11.1.30.
Hemodynamic Characteristics Regarding Recanalization of Completely Coiled Aneurysms: Computational Fluid Dynamic Analysis Using Virtual Models Comparison
- Affiliations
-
- 1Department of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. dcsuh@amc.seoul.kr
- 2Department of Neurosurgery, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- KMID: 2155716
- DOI: http://doi.org/10.5469/neuroint.2016.11.1.30
Abstract
- PURPOSE
Hemodynamic factors are considered to play an important role in initiation and progression of the recurrence after endosaccular coiling of the intracranial aneurysms. We made paired virtual models of completely coiled aneurysms which were subsequently recanalized and compared to identify hemodynamic characteristics related to the recurred aneurysmal sac.
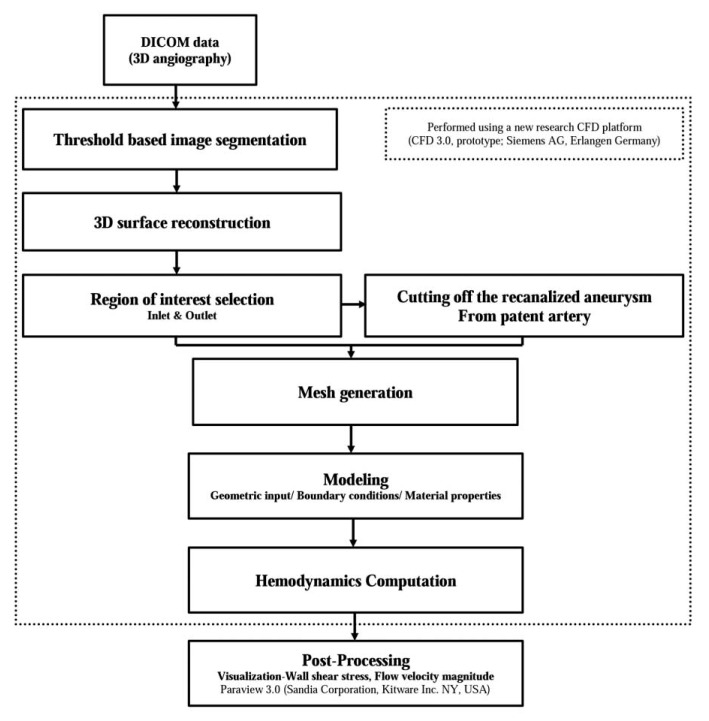
MATERIALS AND METHODS
We created paired virtual models of computational fluid dynamics (CFD) in five aneurysms which were initially regarded as having achieved complete occlusion and then recurred during follow-up. Paired virtual models consisted of the CFD model of 3D rotational angiography obtained in the recurred aneurysm and the control model of the initial, parent artery after artificial removal of the coiled and recanalized aneurysm. Using the CFD analysis of the virtual model, we analyzed the hemodynamic characteristics on the neck of each aneurysm before and after its recurrence.
RESULTS
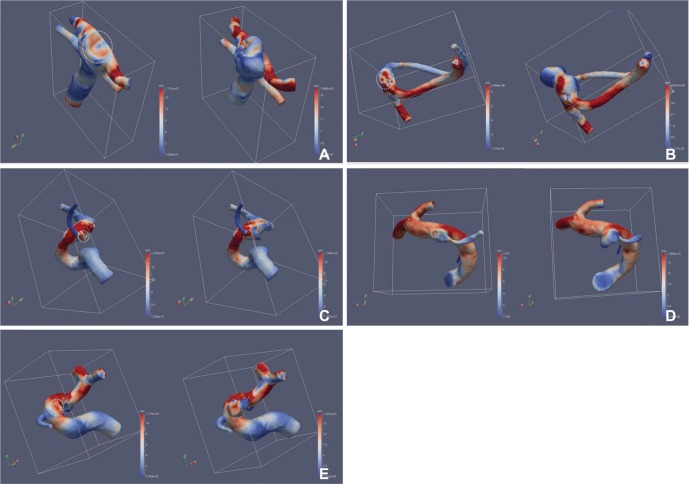
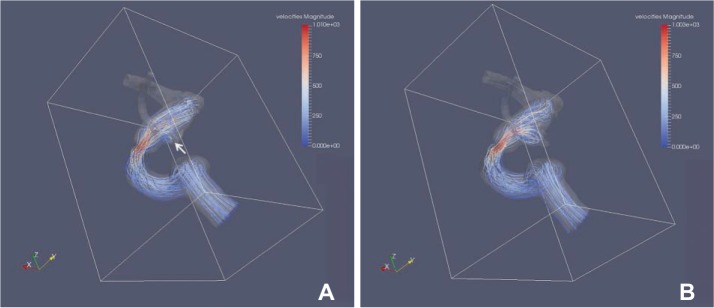
High wall shear stress (WSS) was identified at the cross-sectionally identified aneurysm neck at which recurrence developed in all cases. A small vortex formation with relatively low velocity in front of the neck was also identified in four cases. The aneurysm recurrence locations corresponded to the location of high WSS and/or small vortex formation.
CONCLUSION
Recanalized aneurysms revealed increased WSS and small vortex formation at the cross-sectional neck of the aneurysm. This observation may partially explain the hemodynamic causes of future recanalization after coil embolization.
MeSH Terms
Figure
Cited by 2 articles
-
Microguidewire Looping to Traverse Stented Parent Arteries of Intracranial Aneurysms
Young Dae Cho, Jong Kook Rhim, Dong Hyun Yoo, Hyun-Seung Kang, Jeong Eun Kim, Moon Hee Han
J Korean Neurosurg Soc. 2017;60(2):262-268. doi: 10.3340/jkns.2016.0707.009.Physiologic Flow Diversion Coiling Technique for Wide-Necked Aneurysms with an Asymmetric Bidirectional Flow at the Aneurysm Neck
Boseong Kwon, Yunsun Song, Yun Hyeok Choi, Dae Chul Suh
Neurointervention. 2022;17(3):133-142. doi: 10.5469/neuroint.2022.00311.
Reference
-
1. Cognard C, Weill A, Spelle L, Piotin M, Castaings L, Rey A, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology. 1999; 212:348–356. PMID: 10429689.
Article2. Ng P, Khangure MS, Phatouros CC, Bynevelt M, ApSimon H, McAuliffe W. Endovascular treatment of intracranial aneurysms with Guglielmi detachable coils: analysis of midterm angiographic and clinical outcomes. Stroke. 2002; 33:210–217. PMID: 11779912.3. Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003; 34:1398–1403. PMID: 12775880.
Article4. Park SH, Lee CY, Yim MB. The merits of endovascular coil surgery for patients with unruptured intracranial aneurysms. J Korean Neurosurg Soc. 2008; 43:270–274. PMID: 19096631.
Article5. Grunwald IQ, Papanagiotou P, Struffert T, Politi M, Krick C, Gul G, et al. Recanalization after endovascular treatment of intracerebral aneurysms. Neuroradiology. 2007; 49:41–47. PMID: 17094000.
Article6. Boet R, Wong GK, Poon WS, Lam JM, Yu SC. Aneurysm recurrence after treatment of paraclinoid/ophthalmic segment aneurysms--a treatment-modality assessment. Acta Neurochir (Wien). 2005; 147:611–616. discussion 616PMID: 15806326.7. Ortiz R, Stefanski M, Rosenwasser R, Veznedaroglu E. Cigarette smoking as a risk factor for recurrence of aneurysms treated by endosaccular occlusion. J Neurosurg. 2008; 108:672–675. PMID: 18377244.
Article8. Plowman RS, Clarke A, Clarke M, Byrne JV. Sixteen-year single-surgeon experience with coil embolization for ruptured intracranial aneurysms: recurrence rates and incidence of late rebleeding. Clinical article. J Neurosurg. 2011; 114:863–874. PMID: 20672900.9. Byrne JV, Sohn MJ, Molyneux AJ, Chir B. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg. 1999; 90:656–663. PMID: 10193610.
Article10. Luo B, Yang X, Wang S, Li H, Chen J, Yu H, et al. High shear stress and flow velocity in partially occluded aneurysms prone to recanalization. Stroke. 2011; 42:745–753. PMID: 21233477.
Article11. Lecler A, Raymond J, Rodriguez-Regent C, Al Shareef F, Trystram D, Godon-Hardy S, et al. Intracranial Aneurysms: Recurrences More than 10 Years after Endovascular Treatment-A Prospective Cohort Study, Systematic Review, and Meta-Analysis. Radiology. 2015; 277:173–180. PMID: 26057784.
Article12. Li C, Wang S, Chen J, Yu H, Zhang Y, Jiang F, et al. Influence of hemodynamics on recanalization of totally occluded intracranial aneurysms: a patient-specific computational fluid dynamic simulation study. J Neurosurg. 2012; 117:276–283. PMID: 22680247.
Article13. Ortega J, Hartman J, Rodriguez J, Maitland D. Post-treatment hemodynamics of a basilar aneurysm and bifurcation. Ann Biomed Eng. 2008; 36:1531–1546. PMID: 18629647.
Article14. Irie K, Anzai H, Kojima M, Honjo N, Ohta M, Hirose Y, et al. Computational fluid dynamic analysis following recurrence of cerebral aneurysm after coil embolization. Asian J Neurosurg. 2012; 7:109. PMID: 23293665.
Article15. Liu J, Jing L, Wang C, Paliwal N, Wang S, Zhang Y, et al. Effect of hemodynamics on outcome of subtotally occluded paraclinoid aneurysms after stent-assisted coil embolization. J Neurointerv Surg. 2015; 11. 26. [Epub ahead of print]. DOI: 10.1136/neurintsurg-2015-012050.
Article16. Song Y, Choe J, Liu H, Park KJ, Yu H, Lim OK, et al. Virtual stenting of intracranial aneurysms: application of hemodynamic modification analysis. Acta Radiol. 2015; 10. 25. [Epub ahead of print].
Article17. He X, Duckwiler G, Valentino DJ. Lattice Boltzmann simulation of cerebral artery hemodynamics. Comput Fluids. 2009; 38:789–796.
Article18. Golbert DR, Blanco PJ, Clausse A, Feijoo RA. Tuning a lattice-Boltzmann model for applications in computational hemodynamics. Med Eng Phys. 2012; 34:339–349. PMID: 21880536.
Article19. Mitsos AP, Kakalis NM, Ventikos YP, Byrne JV. Haemodynamic simulation of aneurysm coiling in an anatomically accurate computational fluid dynamics model: technical note. Neuroradiology. 2008; 50:341–347. PMID: 18043912.
Article20. Gallas S, Januel AC, Pasco A, Drouineau J, Gabrillargues J, Gaston A, et al. Long-term follow-up of 1036 cerebral aneurysms treated by bare coils: a multicentric cohort treated between 1998 and 2003. AJNR Am J Neuroradiol. 2009; 30:1986–1992. PMID: 19679641.
Article21. Cha KS, Balaras E, Lieber BB, Sadasivan C, Wakhloo AK. Modeling the interaction of coils with the local blood flow after coil embolization of intracranial aneurysms. J Biomech Eng. 2007; 129:873–879. PMID: 18067391.
Article22. Cebral JR, Lohner R. Efficient simulation of blood flow past complex endovascular devices using an adaptive embedding technique. IEEE Trans Med Imaging. 2005; 24:468–476. PMID: 15822805.
Article23. Groden C, Laudan J, Gatchell S, Zeumer H. Three-dimensional pulsatile flow simulation before and after endovascular coil embolization of a terminal cerebral aneurysm. J Cereb Blood Flow Metab. 2001; 21:1464–1471. PMID: 11740208.
Article24. Gauvrit JY, Leclerc X, Caron S, Taschner CA, Lejeune JP, Pruvo JP. Intracranial aneurysms treated with Guglielmi detachable coils imaging follow-up with contrast-enhanced MR angiography. Stroke. 2006; 37:1033–1037. PMID: 16528000.25. Schaafsma JD, Velthuis BK, Majoie CBLM, van den Berg R, Brouwer PA, Barkhof F, et al. Intracranial aneurysms treated with coil placement: test characteristics of follow-up MR angiography--multicenter study. Radiology. 2010; 256:209–218. PMID: 20505063.
Article26. Missler U, Hundt C, Wiesmann M, Mayer T, Brückmann H. Three-dimensional reconstructed rotational digital subtraction angiography in planning treatment of intracranial aneurysms. Eur Radiol. 2000; 10:564–568. PMID: 10795532.
Article27. Meng H, Wang Z, Hoi Y, Gao L, Metaxa E, Swartz DD, et al. Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke. 2007; 38:1924–1931. PMID: 17495215.
Article28. Chatziprodromou I, Tricoli A, Poulikakos D, Ventikos Y. Haemodynamics and wall remodelling of a growing cerebral aneurysm: a computational model. J Biomech. 2007; 40:412–426. PMID: 16527284.
Article29. Wang J, Tan HQ, Zhu YQ, Li MH, Li ZZ, Yan L, et al. Complex hemodynamic insult in combination with wall degeneration at the apex of an arterial bifurcation contributes to generation of nascent aneurysms in a canine model. AJNR Am J Neuroradiol. 2014; 35:1805–1812. PMID: 24788130.
Article30. Byun HS, Rhee K. CFD modeling of blood flow following coil embolization of aneurysms. Med Eng Phys. 2004; 26:755–761. PMID: 15564112.
Article31. Nakatani H, Hashimoto N, Kang Y, Yamazoe N, Kikuchi H, Yamaguchi S, et al. Cerebral blood flow patterns at major vessel bifurcations and aneurysms in rats. J Neurosurg. 1991; 74:258–262. PMID: 1988596.
Article32. Mantha A, Karmonik C, Benndorf G, Strother C, Metcalfe R. Hemodynamics in a cerebral artery before and after the formation of an aneurysm. AJNR Am J Neuroradiol. 2006; 27:1113–1118. PMID: 16687554.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Current Limitations and Advanced Analysis of Hemodynamic Study of Cerebral Aneurysms
- Influence of Parent Artery Segmentation and Boundary Conditions on Hemodynamic Characteristics of Intracranial Aneurysms
- Patient-Specific Computational Fluid Dynamics in Ruptured Posterior Communicating Aneurysms Using Measured Non-Newtonian Viscosity : A Preliminary Study
- Reappraisal of Anatomic Outcome Scales of Coiled Intracranial Aneurysms in the Prediction of Recanalization
- Computational Fluid Dynamics: Comparison of Prototype and Commercial Solutions for Intracranial Aneurysms with Different Entrance Length