J Korean Med Sci.
2015 May;30(5):625-631. 10.3346/jkms.2015.30.5.625.
Functional Magnetic Resonance Imaging of Motor Cortex Activation in Schizophrenia
- Affiliations
-
- 1Division of Computer Science and Engineering, CAIIT, Chonbuk National University, Jeonju, Korea. hlee@chonbuk.ac.kr
- 2Department of Psychiatry and Human Behavior, University of California, Irvine, CA, USA.
- 3Department of Psychiatry, San Francisco VAMC and University of California, San Francisco, CA, USA.
- 4Department of Psychology and Neuroscience, Georgia State University, Atlanta, GA, USA.
- KMID: 2155477
- DOI: http://doi.org/10.3346/jkms.2015.30.5.625
Abstract
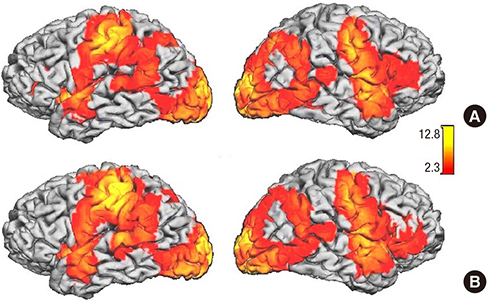
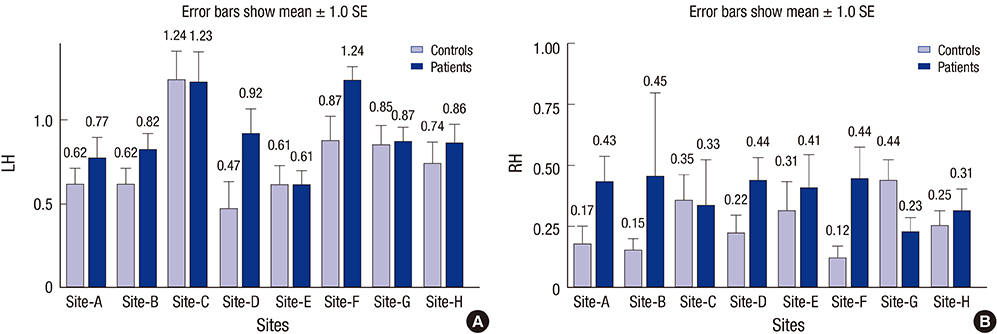
- Previous fMRI studies of sensorimotor activation in schizophrenia have found in some cases hypoactivity, no difference, or hyperactivity when comparing patients with controls; similar disagreement exists in studies of motor laterality. In this multi-site fMRI study of a sensorimotor task in individuals with chronic schizophrenia and matched healthy controls, subjects responded with a right-handed finger press to an irregularly flashing visual checker board. The analysis includes eighty-five subjects with schizophrenia diagnosed according to the DSM-IV criteria and eighty-six healthy volunteer subjects. Voxel-wise statistical parametric maps were generated for each subject and analyzed for group differences; the percent Blood Oxygenation Level Dependent (BOLD) signal changes were also calculated over predefined anatomical regions of the primary sensory, motor, and visual cortex. Both healthy controls and subjects with schizophrenia showed strongly lateralized activation in the precentral gyrus, inferior frontal gyrus, and inferior parietal lobule, and strong activations in the visual cortex. There were no significant differences between subjects with schizophrenia and controls in this multi-site fMRI study. Furthermore, there was no significant difference in laterality found between healthy controls and schizophrenic subjects. This study can serve as a baseline measurement of schizophrenic dysfunction in other cognitive processes.
Keyword
MeSH Terms
Figure
Reference
-
1. Mamah D, Wang L, Barch D, de Erausquin GA, Gado M, Csernansky JG. Structural analysis of the basal ganglia in schizophrenia. Schizophr Res. 2007; 89:59–71.2. Jung WH, Jang JH, Byun MS, An SK, Kwon JS. Structural brain alterations in individuals at ultra-high risk for psychosis: a review of magnetic resonance imaging studies and future directions. J Korean Med Sci. 2010; 25:1700–1709.3. Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999; 156:1358–1366.4. Günther W, Petsch R, Steinberg R, Moser E, Streck P, Heller H, Kurtz G, Hippius H. Brain dysfunction during motor activation and corpus callosum alterations in schizophrenia measured by cerebral blood flow and magnetic resonance imaging. Biol Psychiatry. 1991; 29:535–555.5. Nuechterlein KH. Reaction time and attention in schizophrenia: a critical evaluation of the data and theories. Schizophr Bull. 1977; 3:373–428.6. Vrtunski PB, Simpson DM, Meltzer HY. Voluntary movement dysfunction in schizophrenics. Biol Psychiatry. 1989; 25:529–539.7. Singh J, Knight RT, Rosenlicht N, Kotun JM, Beckley DJ, Woods DL. Abnormal premovement brain potentials in schizophrenia. Schizophr Res. 1992; 8:31–41.8. Schröder J, Wenz F, Schad LR, Baudendistel K, Knopp MV. Sensorimotor cortex and supplementary motor area changes in schizophrenia. A study with functional magnetic resonance imaging. Br J Psychiatry. 1995; 167:197–201.9. Mattay VS, Callicott JH, Bertolino A, Santha AK, Tallent KA, Goldberg TE, Frank JA, Weinberger DR. Abnormal functional lateralization of the sensorimotor cortex in patients with schizophrenia. Neuroreport. 1997; 8:2977–2984.10. Günther W, Brodie JD, Bartlett EJ, Dewey SL, Henn FA, Volkow ND, Alper K, Wolkin A, Cancro R, Wolf AP. Diminished cerebral metabolic response to motor stimulation in schizophrenics: a PET study. Eur Arch Psychiatry Clin Neurosci. 1994; 244:115–125.11. Müller JL, Röder C, Schuierer G, Klein HE. Subcortical overactivation in untreated schizophrenic patients: a functional magnetic resonance image finger-tapping study. Psychiatry Clin Neurosci. 2002; 56:77–84.12. Rogowska J, Gruber SA, Yurgelun-Todd DA. Functional magnetic resonance imaging in schizophrenia: cortical response to motor stimulation. Psychiatry Res. 2004; 130:227–243.13. Honey GD, Pomarol-Clotet E, Corlett PR, Honey RA, McKenna PJ, Bullmore ET, Fletcher PC. Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005; 128:2597–2611.14. Braus DF, Ende G, Hubrich-Ungureanu P, Henn FA. Cortical response to motor stimulation in neuroleptic-naive first episode schizophrenics. Psychiatry Res. 2000; 98:145–154.15. Buckley PF, Friedman L, Wu D, Lai S, Meltzer HY, Haacke EM, Miller D, Lewin JS. Functional magnetic resonance imaging in schizophrenia: initial methodology and evaluation of the motor cortex. Psychiatry Res. 1997; 74:13–23.16. Mager T, Weilke FA, Leinsinger GL, Dudel C, Heiss D, Günther W, Ulbricht D, Hahn K, Möller HJ. Activation of the motor cortex in schizophrenics investigated by functional MR imaging. Neuroimage. 1996; 3:S497.17. Schröder J, Niethammer R, Geider FJ, Reitz C, Binkert M, Jauss M, Sauer H. Neurological soft signs in schizophrenia. Schizophr Res. 1991; 6:25–30.18. Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A 3rd, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001; 58:280–288.19. Keator DB, Grethe JS, Marcus D, Ozyurt B, Gadde S, Murphy S, Pieper S, Greve D, Notestine R, Bockholt HJ, et al. BIRN Function. BIRN Morphometry. BIRN-Coordinating. A national human neuroimaging collaboratory enabled by the Biomedical Informatics Research Network (BIRN). IEEE Trans Inf Technol Biomed. 2008; 12:162–172.20. Potkin SG, Ford JM. Widespread cortical dysfunction in schizophrenia: the FBIRN imaging consortium. Schizophr Bull. 2009; 35:15–18.21. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004; 23:S208–S219.22. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002; 17:825–841.23. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002; 17:143–155.24. Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004; 21:1732–1747.25. Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000; 10:120–131.26. Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000; 12:191–200.27. Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003; 19:1233–1239.28. Bertolino A, Blasi G, Caforio G, Latorre V, De Candia M, Rubino V, Callicott JH, Mattay VS, Bellomo A, Scarabino T, et al. Functional lateralization of the sensorimotor cortex in patients with schizophrenia: effects of treatment with olanzapine. Biol Psychiatry. 2004; 56:190–197.29. Fernández G, Weis S, Stoffel-Wagner B, Tendolkar I, Reuber M, Beyenburg S, Klaver P, Fell J, de Greiff A, Ruhlmann J, et al. Menstrual cycle-dependent neural plasticity in the adult human brain is hormone, task, and region specific. J Neurosci. 2003; 23:3790–3795.30. Lent RV. Java applet for power and sample size. accessed on 10 September 2014. Available at http://homepage.stat.uiowa.edu/~rlenth/Power/.31. Wexler BE, Hawkins KA, Rounsaville B, Anderson M, Sernyak MJ, Green MF. Normal neurocognitive performance after extended practice in patients with schizophrenia. Schizophr Res. 1997; 26:173–180.32. McFarland K, Anderson J. Factor stability of the Edinburgh Handedness Inventory as a function of test-retest performance, age and sex. Br J Psychol. 1980; 71:135–142.33. Blair JR, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. Clin Neuropsychol. 1989; 3:129–136.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Functional Magnetic Resonance Imaging during Motor Activation in Patients with Schizophrenia
- Disturbed Functional Asymmetry of Sensorimotor Cortex in Schizophrenia: A Study with Functional Magnetic Resonance Imaging
- Functional MR Imaging of the Motor Cortex in Active and Passive Movement: Qualitative and Quantitative Changes
- Cortical Activation Related to Motor and Sensory Tasks in Congenital Mirror Movement using Functional MRI
- Functional MR Imaging of Cerbral Motor Cortex: Comparison between Conventional Gradient Echo and EPI Techniques