Impact of Molecular Subtype Conversion of Breast Cancers after Neoadjuvant Chemotherapy on Clinical Outcome
- Affiliations
-
- 1Center for Breast Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Korea. eslee@ncc.re.kr
- 2Breast Service, Department of General Surgery, Changi General Hospital, Singapore.
- 3Cancer Biostatistics Branch, Research Institute for National Cancer Control and Evaluation, Research Institute and Hospital, National Cancer Center, Goyang, Korea.
- KMID: 2152269
- DOI: http://doi.org/10.4143/crt.2014.262
Abstract
- PURPOSE
The aim of this study was to examine molecular subtype conversions in patients who underwent neoadjuvant chemotherapy (NAC) and analyze their clinical implications.
MATERIALS AND METHODS
We included consecutive breast cancer patients who received NAC at the National Cancer Center, Korea, between August 2002 and June 2011, and had available data on estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor 2 (HER2) receptor status prior to NAC. Molecular subtypes, hormone receptor (HR) status, and ER and PR Allred scores before and after NAC were compared, and the long-term outcomes were analyzed.
RESULTS
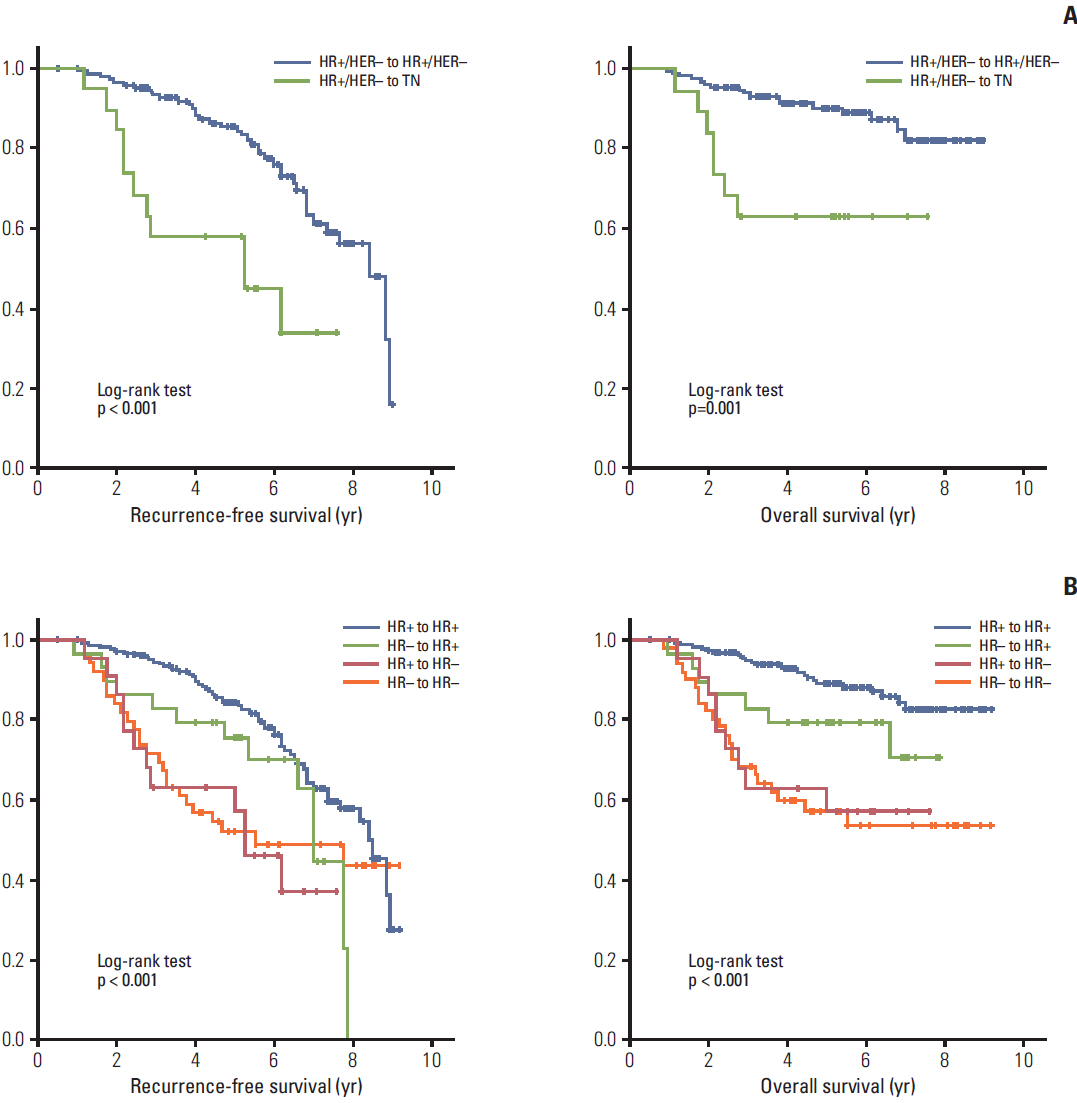
Of 322 patients, 32 (9.9%) achieved a pathologic complete response after NAC. HR+/HER2- tumors tended to convert into triple negative (TN) tumors (10.3%), whereas 34.6% of TN tumors gained HR positivity to become HR+/HER2- tumors. Clinical outcomes of molecular subtype conversion groups were compared against patients who remained as HR+/HER2- throughout. The HR+/HER2- to TN group had significantly poorer recurrence-free survival (RFS) (hazard ratio, 3.54; 95% confidence interval [CI], 1.60 to 7.85) and overall survival (OS) (hazard ratio, 3.73; 95% CI, 1.34 to 10.38). Patients who remained TN throughout had the worst outcomes (for RFS: hazard ratio, 3.70; 95% CI, 1.86 to 7.36; for OS: hazard ratio, 5.85; 95% CI, 2.53 to 13.51), while those who converted from TN to HR+/HER2-showed improved comparable survival outcomes.
CONCLUSION
Molecular subtypes of breast cancers changed frequently after NAC, resulting in different tumor prognostication. Tumor subtyping should be repeated after NAC in patients with breast cancer.
MeSH Terms
Figure
Cited by 4 articles
-
Predictive and Prognostic Roles of Pathological Indicators for Patients with Breast Cancer on Neoadjuvant Chemotherapy
Xinyan Li, Mozhi Wang, Mengshen Wang, Xueting Yu, Jingyi Guo, Tie Sun, Litong Yao, Qiang Zhang, Yingying Xu
J Breast Cancer. 2019;22(4):497-521. doi: 10.4048/jbc.2019.22.e49.HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation
Soomin Ahn, Ji Won Woo, Kyoungyul Lee, So Yeon Park
J Pathol Transl Med. 2020;54(1):34-44. doi: 10.4132/jptm.2019.11.03.Negative Conversion of Progesterone Receptor Status after Primary Systemic Therapy Is Associated with Poor Clinical Outcome in Patients with Breast Cancer
Soomin Ahn, Hyun Jeong Kim, Milim Kim, Yul Ri Chung, Eunyoung Kang, Eun-Kyu Kim, Se Hyun Kim, Yu Jung Kim, Jee Hyun Kim, In Ah Kim, So Yeon Park
Cancer Res Treat. 2018;50(4):1418-1432. doi: 10.4143/crt.2017.552.Molecular Classification of Breast Cancer Using Weakly Supervised Learning
Wooyoung Jang, Jonghyun Lee, Kyong Hwa Park, Aeree Kim, Sung Hak Lee, Sangjeong Ahn
Cancer Res Treat. 2025;57(1):116-125. doi: 10.4143/crt.2024.113.
Reference
-
References
1. Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001; 30:96–102.
Article2. Chen AM, Meric-Bernstam F, Hunt KK, Thames HD, Oswald MJ, Outlaw ED, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson Cancer Center experience. J Clin Oncol. 2004; 22:2303–12.
Article3. Mittendorf EA, Buchholz TA, Tucker SL, Meric-Bernstam F, Kuerer HM, Gonzalez-Angulo AM, et al. Impact of chemotherapy sequencing on local-regional failure risk in breast cancer patients undergoing breast-conserving therapy. Ann Surg. 2013; 257:173–9.
Article4. Zhang N, Moran MS, Huo Q, Haffty BG, Yang Q, et al. The hormonal receptor status in breast cancer can be altered by neoadjuvant chemotherapy: a meta-analysis. Cancer Invest. 2011; 29:594–8.
Article5. van de Ven S, Smit VT, Dekker TJ, Nortier JW, Kroep JR. Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev. 2011; 37:422–30.
Article6. Tacca O, Penault-Llorca F, Abrial C, Mouret-Reynier MA, Raoelfils I, Durando X, et al. Changes in and prognostic value of hormone receptor status in a series of operable breast cancer patients treated with neoadjuvant chemotherapy. Oncologist. 2007; 12:636–43.
Article7. Hirata T, Shimizu C, Yonemori K, Hirakawa A, Kouno T, Tamura K, et al. Change in the hormone receptor status following administration of neoadjuvant chemotherapy and its impact on the long-term outcome in patients with primary breast cancer. Br J Cancer. 2009; 101:1529–36.
Article8. Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013; 381:805–16.9. Zardavas D, Ades F, de Azambuja E. Clinical practice-changing trials: the HERA study paradigm. Expert Rev Anticancer Ther. 2013; 13:1249–56.
Article10. Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005; 11:5678–85.
Article11. von Minckwitz G, Fontanella C. Selecting the neoadjuvant treatment by molecular subtype: how to maximize the benefit? Breast. 2013; 22 Suppl 2:S149–51.
Article12. Schnitt SJ. Classification and prognosis of invasive breast cancer: from morphology to molecular taxonomy. Mod Pathol. 2010; 23 Suppl 2:S60–4.
Article13. Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006; 295:2492–502.
Article14. Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009; 7:4–13.
Article15. Lee S, Kim SW, Kim SK, Lee KS, Kim EA, Kwon Y, et al. Locoregional recurrence of breast conserving surgery after preoperative chemotherapy in korean women with locally advanced breast cancer. J Breast Cancer. 2011; 14:289–95.
Article16. Lee KS, Ro J, Lee ES, Kang HS, Kim SW, Nam BH, et al. Primary systemic therapy with intermittent weekly paclitaxel plus gemcitabine in patients with stage II and III breast cancer: a phase II trial. Invest New Drugs. 2010; 28:83–90.
Article17. Im SA, Lee KS, Ro J, Lee ES, Kwon Y, Ahn JH, et al. Phase II trial of preoperative paclitaxel, gemcitabine, and trastuzumab combination therapy in HER2 positive stage II/III breast cancer: the Korean Cancer Study Group BR 07-01. Breast Cancer Res Treat. 2012; 132:589–600.
Article18. Park IH, Lee KS, Kang HS, Kim SW, Lee S, Jung SY, et al. A phase Ib study of preoperative lapatinib, paclitaxel, and gemcitabine combination therapy in women with HER2 positive early breast cancer. Invest New Drugs. 2012; 30:1972–7.
Article19. Tomasello G, de Azambuja E, Dinh P, Snoj N, Piccart-Gebhart M. Jumping higher: is it still possible? The ALTTO trial challenge. Expert Rev Anticancer Ther. 2008; 8:1883–90.
Article20. Kaufmann M, Hortobagyi GN, Goldhirsch A, Scholl S, Makris A, Valagussa P, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol. 2006; 24:1940–9.
Article21. Chen S, Chen CM, Yu KD, Zhou RJ, Shao ZM. Prognostic value of a positive-to-negative change in hormone receptor status after neoadjuvant chemotherapy in patients with hormone receptor-positive breast cancer. Ann Surg Oncol. 2012; 19:3002–11.
Article22. Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010; 28:1684–91.
Article23. Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012; 48:3342–54.
Article24. Kawajiri H, Takashima T, Aomatsu N, Kashiwagi S, Noda S, Onoda N, et al. Prognostic significance of pathological complete response following neoadjuvant chemotherapy for operable breast cancer. Oncol Lett. 2014; 7:663–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impact of Immunohistochemistry-Based Molecular Subtype on Chemosensitivity and Survival in Patients with Breast Cancer Following Neoadjuvant Chemotherapy
- Prognostic Factors in Patients with Locally Advanced Breast Cancer Treated by Neoadjuvant Chemotherapy
- Bilateral Triple Negative Invasive Ductal Breast Carcinoma in a BRCA1 Mutation Carrier with Discrepant Pathologic Response to Neoadjuvant Chemotherapy
- Correlation between Tumor Response to Neoadjuvant Chemotherapy and Patient Outcome in Breast Cancer
- Potential Benefits of Neoadjuvant Chemotherapy in Clinically Node-Positive Luminal Subtypeâ» Breast Cancer