J Korean Med Sci.
2010 Jun;25(6):905-911. 10.3346/jkms.2010.25.6.905.
Increased Seizure Susceptibility and Up-regulation of nNOS Expression in Hippocampus Following Recurrent Early-life Seizures in Rats
- Affiliations
-
- 1Department of Pediatrics, Dongguk University College of Medicine, Gyeongju, Korea. pedepi@hotmail.com
- KMID: 2150871
- DOI: http://doi.org/10.3346/jkms.2010.25.6.905
Abstract
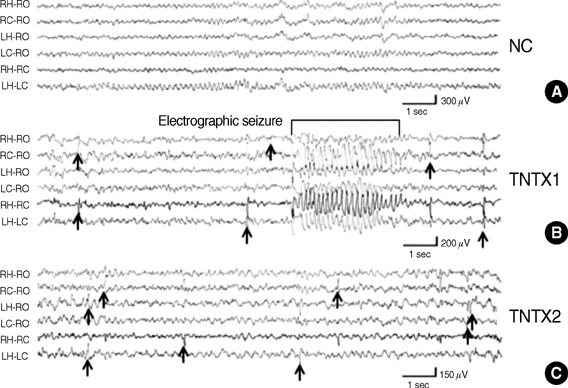
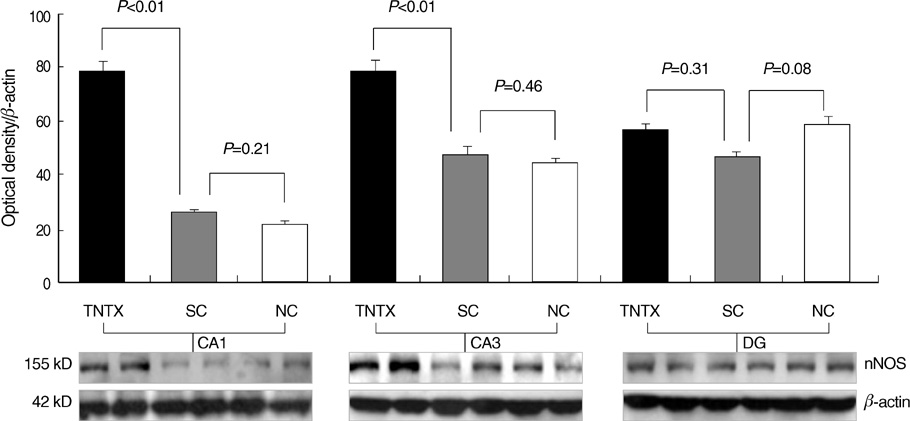
- This study aimed to determine the long-term change of seizure susceptibility and the role of nNOS on brain development following recurrent early-life seizures in rats. Video-EEG recordings were conducted between postnatal days 50 and 60. Alterations in seizure susceptibility were assayed on day 22 or 50 using the flurothyl method. Changes in nNOS expression were determined by quantitative immunoblotting on day 50. On average, rats had 8.4+/-2.7 seizures during 10 daily 1 hr behavioral monitoring sessions. As adults (days 50-60), all rats displayed interictal spikes in the hippocampus and/or overlying cortex. Brief electrographic seizures were recorded in only one of five animals. Rats appeared to progress from a period of marked seizure susceptibility (day 22) to one of lessened seizure susceptibility (day 50). Up-regulation of nNOS expression following early-life recurrent seizures was observed on day 50. In conclusion, these data suggested that recurrent early-life seizures had the long-term effects on seizure susceptibility late in life and up-regulatory nNOS expression on the hippocampus during brain development, and nNOS appeared to contribute to the persistent changes in seizure susceptibility, and epileptogenesis.
Keyword
Figure
Reference
-
1. Jensen FE. Acute and chronic effects of seizures in the developing brain: experimental models. Epilepsia. 1999. 40:Suppl 1. S51–S58.2. Holmes GL. Epilepsy in the developing brain: lessons from the laboratory and clinic. Epilepsia. 1997. 38:12–30.3. Holmes GL, Khazipov R, Ben-Ari Y. New concepts in neonatal seizures. Neuroreport. 2002. 21:A3–A8.4. Swann JW, Pierson MG, Smith KL, Lee CL. Developmental neuroplasticity: roles in early life seizures and chronic epilepsy. Adv Neurol. 1999. 79:203–216.5. Swann JW, Hablitz JJ. Cellular abnormalities and synaptic plasticity in seizure disorders of the immature nervous system. Ment Retard Dev Disabil Res Rev. 2000. 6:258–267.6. Hawkins CA, Mellanby JH. Limbic epilepsy induced by tetanus toxin: a longitudinal electroencephalographic study. Epilepsia. 1987. 28:431–444.7. Jefferys JG, Williams SF. Physiological and behavioral consequences of seizures induced in the rat by intrahippocampal tetanus toxin. Brain. 1987. 110:517–532.8. Mellanby J, George G, Robinson A, Thompson P. Epileptiform syndrome in rats produced by injecting tetanus toxin into the hippocampus. J Neurol Neurosurg Psychiatry. 1977. 40:404–414.9. Lee CL, Hrachovy RA, Smith KL, Frost JD Jr, Swann JW. Seizures produced in neonatal rats by stereotaxic injections of tetanus toxin (TT): in vivo and in vitro models of seizures in early infancy. Epilepsia. 1993. 34:59.10. Dawson TM, Dawson VL, Snyder SH. A novel neuronal messenger molecule in the brain: the free radical, nitric oxide. Ann Neurol. 1992. 32:297–311.11. Roskams AJ, Bredt DS, Dawson TM, Ronnet GV. Nitric oxide mediates the formation of synaptic connections in developing and regenerating olfactory receptor neurons. Neuron. 1994. 13:289–299.12. Endoh M, Maiese K, Wagner JA. Expression of the neural form of nitric oxide synthase by CA1 hippocampal neurons and other central nervous system neurons. Neuroscience. 1994. 63:679–689.13. Lee CL, Hrachovy RA, Smith KL, Frost JD Jr, Swann JW. Tetanus toxin-induced seizures in infant rats and their effects on hippocampal excitability in adulthood. Brain Res. 1995. 17:97–109.14. Smith KL, Lee CL, Swann JW. Local circuit abnormalities in chronically epileptic rats after intrahippocampal tetanus toxin injection in infancy. J Neurophysiol. 1998. 79:106–116.15. Cha BH, Silveira DC, Liu X, Hu Y, Holmes GL. Effect of topiramate following recurrent and prolonged seizures during early development. Epilepsy Res. 2002. 51:217–232.16. Cavalheiro EA, de Feo MR, Mecarelli O, Ricci GF. Intracortical and intrahippocampal injections of kainic acid in developing rats: an electrographic study. Electroencephalogr Clin Neurophysiol. 1983. 56:480–486.17. Tremblay E, Nitecka L, Berger ML, Ben-Ari Y. Maturation of kainic acid seizure-brain damage syndrome in the rat. I. Clinical electrographic and metabolic observations. Neuroscience. 1984. 13:1051–1072.18. Michelson HB, Lothman EW. An ontogenetic study of kindling using rapidly recurring hippocampal seizures. Brain Res Dev Brain Res. 1991. 61:79–85.19. Moshé SL, Albala BJ. Kindling in developing rats: persistence of seizures into adulthood. Brain Res. 1982. 256:67–71.20. Haut SR, Velísková J, Moshé SL. Susceptibility of immature and adult brains to seizure effects. Lancet Neurol. 2004. 3:608–617.21. Baram TZ, Hirsch E, Schultz L. Short interval amygdale kindling in neonatal rats. Brain Res Dev Brain Res. 1993. 73:79–83.22. Albus U, Habermann E. Tetanus toxin inhibits the evoked outflow of inhibitory (GABA) and excitatory (o-aspartate) amino acid from particulate brain cortex. Toxicon. 1983. 21:97–110.23. Williamson LC, Fitzgerald SC, Neale EA. Differential effects of tetanus toxin on inhibitory and excitatory neurotransmitter release from mammalian spinal cord cells in culture. J Neurochem. 1992. 59:2148–2157.24. Jefferys JG. Chronic epileptic foci in vitro in hippocampal slices from rats with tetanus toxin epileptic syndrome. J Neurophysiol. 1989. 62:458–468.25. Haas KZ, Sperber EF, Moshé SL. Kindling in developing animals: expression of severe seizures and enhanced development of bilateral foci. Brain Res Dev Brain Res. 1990. 56:275–280.26. Swann JW, Al-Noori S, Jiang M, Lee CL. Spine loss and other dendritic abnormalities in epilepsy. Hippocampus. 2000. 10:617–625.27. Elmér E, Alm P, Kokaia Z, Kokaia M, Larsson B, Keep M, Andersson KE, Lindvall O. Regulation of neuronal nitric oxide synthase mRNA levels in rat brain by seizure activity. Neuroreport. 1996. 7:1335–1339.28. Ohyu J, Takashima S. Developmental characteristics of neuronal nitric oxide synthase (nNOS) immunoreactive neurons in fetal to adolescent human brains. Brain Res Dev Brain Res. 1998. 110:193–202.29. Bonthius DJ, Tzouras G, Karacay B, Mahoney J, Hutton A, McKim R, Pantazis NJ. Deficiency of neuronal nitric oxide synthase (nNOS) worsens alcohol-induced microencephaly and neuronal loss in developing mice. Brain Res Dev Brain Res. 2002. 138:45–59.30. Sunico CR, Portillo F, Gonzalez-Forero D, Moreno-Lopez B. Nitricoxide-directed synaptic remodeling in the adult mammal CNS. J Neurosci. 2005. 25:1448–1458.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characteristics of Seizures and Neuronal Nitric Oxide Synthase Expression of Hippocampus in Hyperthermia-Induced Seizures of Developing Rat
- Recurrent Early-Life Seizures and Changes in GABAA Receptors Expression in Hippocampus
- The Immunohistochemical Expression of Neuronal Nitric Oxide Synthase in Rat Hippocampus after Pentylenetetrazole-Induced Seizures
- The Role of Nitric Oxide in Seizures Induced by Pentylenetetrazole
- Expressional Change of Nitric Oxide Synthase and erbB4 in Rat Hippocampus after Seizure