Immune Netw.
2015 Oct;15(5):252-259. 10.4110/in.2015.15.5.252.
Altered Gut Microbiota Composition in Rag1-deficient Mice Contributes to Modulating Homeostasis of Hematopoietic Stem and Progenitor Cells
- Affiliations
-
- 1Integrative Biosciences & Biotechnology, Pohang University of Science and Technology (POSTECH), Pohang 37673, Korea. sw_lee@postech.ac.kr
- 2Department of Life Sciences, Pohang University of Science and Technology (POSTECH), Pohang 37673, Korea.
- KMID: 2150854
- DOI: http://doi.org/10.4110/in.2015.15.5.252
Abstract
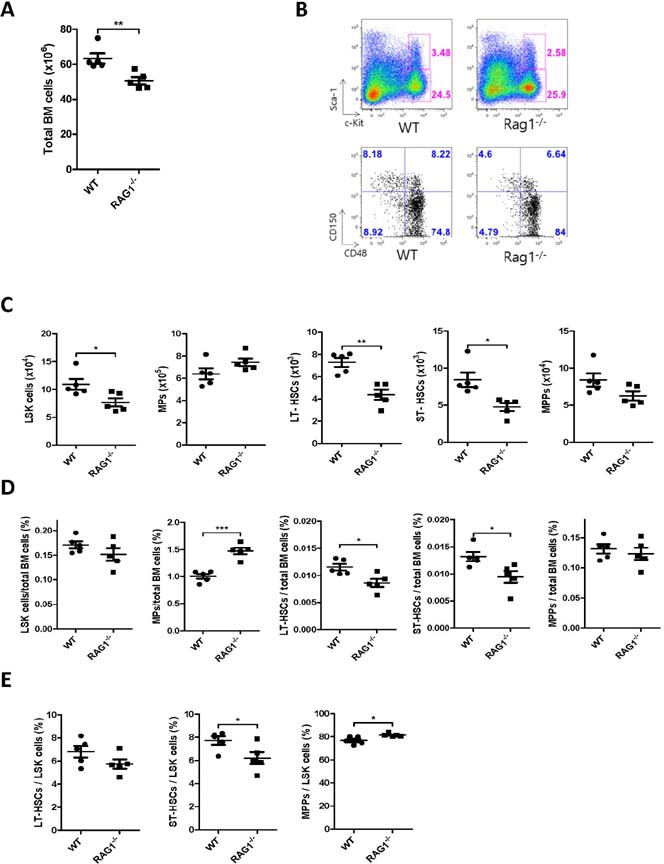
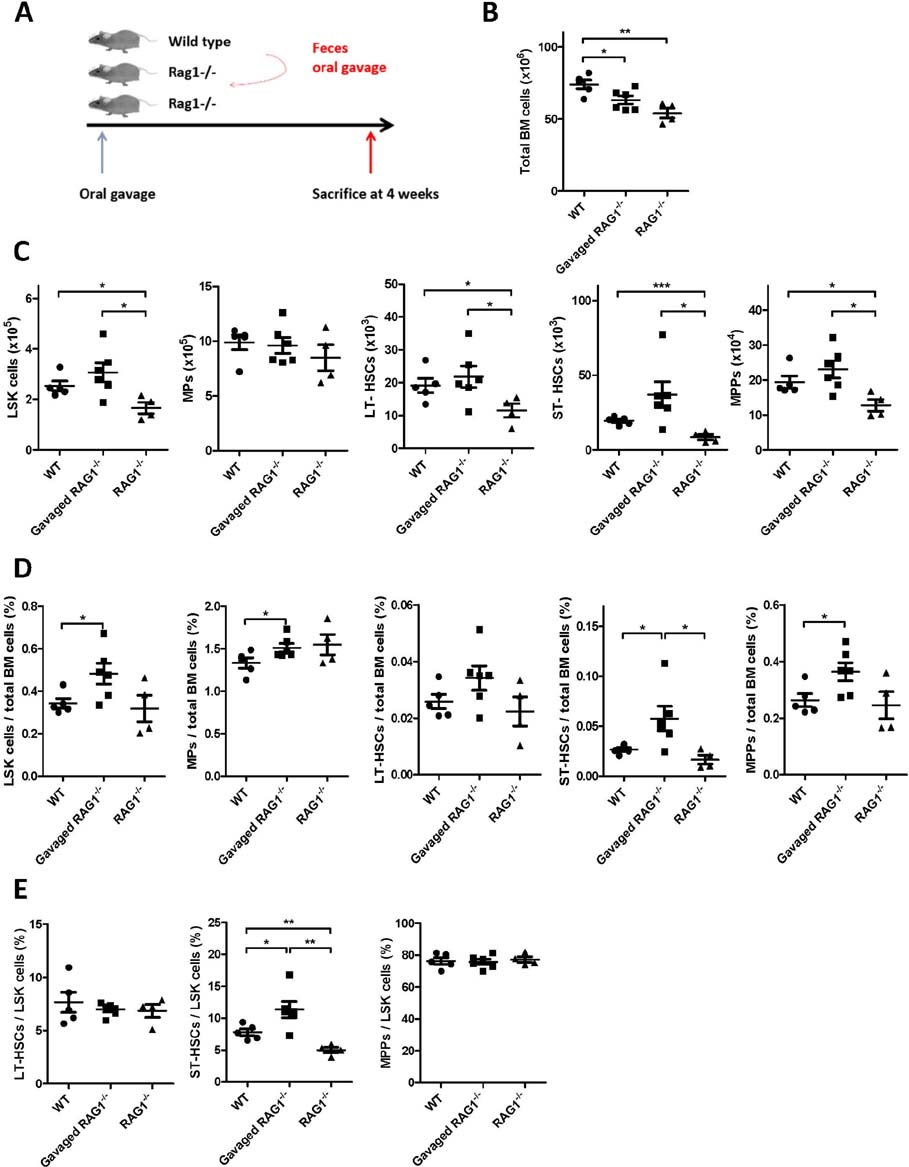
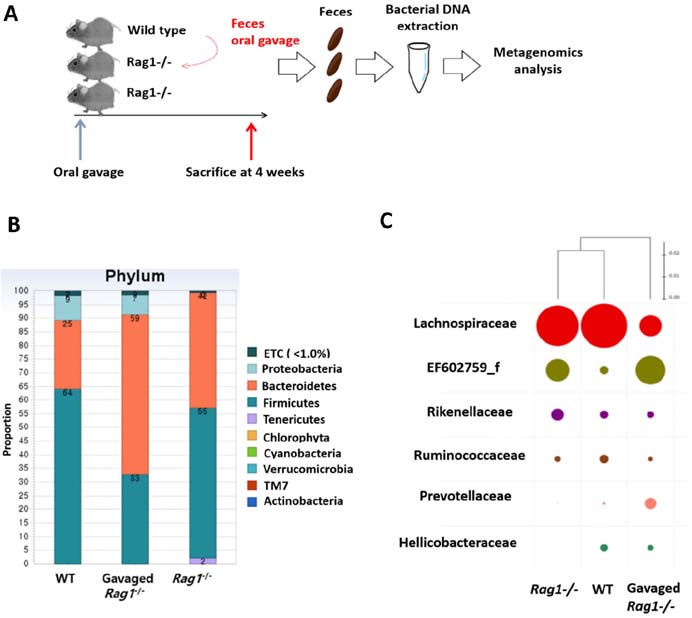
- Hematopoietic stem and progenitor cells (HSPCs) can produce all kind of blood lineage cells, and gut microbiota that consists of various species of microbe affects development and maturation of the host immune system including gut lymphoid cells and tissues. However, the effect of altered gut microbiota composition on homeostasis of HSPCs remains unclear. Here we show that compositional change of gut microbiota affects homeostasis of HSPCs using Rag1(-/-) mice which represent lymphopenic condition. The number and proportions of HSPCs in Rag1(-/-) mice are lower compared to those of wild types. However, the number and proportions of HSPCs in Rag1(-/-) mice are restored as the level of wild types through alteration of gut microbiota diversity via transferring feces from wild types. Gut microbiota composition of Rag1(-/-) mice treated with feces from wild types shows larger proportions of family Prevotellaceae and Helicobacterceae whereas lower proportions of family Lachnospiraceae compared to unmanipulated Rag1(-/-) mice. In conclusion, gut microbiota composition of lymphopenic Rag1(-/-) mice is different to that of wild type, which may lead to altered homeostasis of HSPCs.
Figure
Reference
-
1. Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, Shizuru JA, Weissman IL. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003; 21:759–806.
Article2. Warr MR, Pietras EM, Passegue E. Mechanisms controlling hematopoietic stem cell functions during normal hematopoiesis and hematological malignancies. Wiley Interdiscip Rev Syst Biol Med. 2011; 3:681–701.
Article3. van der Wath RC, Wilson A, Laurenti E, Trumpp A, Lio P. Estimating dormant and active hematopoietic stem cell kinetics through extensive modeling of bromodeoxyuridine label-retaining cell dynamics. PLoS One. 2009; 4:e6972.
Article4. Trumpp A, Essers M, Wilson A. Awakening dormant haematopoietic stem cells. Nat Rev Immunol. 2010; 10:201–209.
Article5. Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006; 6:93–106.
Article6. Arai F, Yoshihara H, Hosokawa K, Nakamura Y, Gomei Y, Iwasaki H, Suda T. Niche regulation of hematopoietic stem cells in the endosteum. Ann N Y Acad Sci. 2009; 1176:36–46.
Article7. Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008; 8:290–301.
Article8. Busch K, Klapproth K, Barile M, Flossdorf M, Holland-Letz T, Schlenner SM, Reth M, Hofer T, Rodewald HR. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature. 2015; 518:542–546.
Article9. Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, Lio P, Macdonald HR, Trumpp A. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008; 135:1118–1129.
Article10. Sommer F, Backhed F. The gut microbiota--masters of host development and physiology. Nat Rev Microbiol. 2013; 11:227–238.
Article11. Lederberg J, Mccray A. 'Ome Sweet 'Omics--A Genealogical Treasury of Words. The Scientist. 2001; 17(7):12. NIH HMP Working Group. Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, Bonazzi V, McEwen JE, Wetterstrand KA, Deal C, Baker CC, Di Francesco V, Howcroft TK, Karp RW, Lunsford RD, Wellington CR, Belachew T, Wright M, Giblin C, David H, Mills M, Salomon R, Mullins C, Akolkar B, Begg L, Davis C, Grandison L, Humble M, Khalsa J, Little AR, Peavy H, Pontzer C, Portnoy M, Sayre MH, Starke-Reed P, Zakhari S, Read J, Watson B, Guyer M. The NIH Human Microbiome Project. Genome Res. 2009; 19:2317–2323.
Article13. Marsland BJ, Gollwitzer ES. Host-microorganism interactions in lung diseases. Nat Rev Immunol. 2014; 14:827–835.
Article14. Park SJ, Namkoong H, Doh J, Choi JC, Yang BG, Park Y, Chul SY. Negative role of inducible PD-1 on survival of activated dendritic cells. J Leukoc Biol. 2014; 95:621–629.
Article15. Khosravi A, Yanez A, Price JG, Chow A, Merad M, Goodridge HS, Mazmanian SK. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014; 15:374–381.
Article16. Zhang H, Sparks JB, Karyala SV, Settlage R, Luo XM. Host adaptive immunity alters gut microbiota. ISME J. 2015; 9:770–781.
Article17. Scholz F, Badgley BD, Sadowsky MJ, Kaplan DH. Immune mediated shaping of microflora community composition depends on barrier site. PLoS One. 2014; 9:e84019.
Article18. Antonopoulos DA, Huse SM, Morrison HG, Schmidt TM, Sogin ML, Young VB. Reproducible community dynamics of the gastrointestinal microbiota following antibiotic perturbation. Infect Immun. 2009; 77:2367–2375.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adult Stem Cells: Beyond Regenerative Tool, More as a Bio-Marker in Obesity and Diabetes
- Gut Microbiota and Metabolic Disorders
- Gut Microbiota in Inflammatory Bowel Disease
- The autophagy Protein Atg5 Plays a Crucial Role in the Maintenance and Reconstitution Ability of Hematopoietic Stem Cells
- The clinical impact of gut microbiota in chronic kidney disease