Immune Netw.
2012 Dec;12(6):269-276. 10.4110/in.2012.12.6.269.
Dendritic Cell (DC) Vaccine in Mouse Lung Cancer Minimal Residual Model; Comparison of Monocyte-derived DC vs. Hematopoietic Stem Cell Derived-DC
- Affiliations
-
- 1Office of Biomedical Professors, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea. andyjosh@skku.edu
- 2Department of Thoracic & Cardiovascular Surgery, Jeju National University School of Medicine, Jeju 690-767, Korea.
- KMID: 2150759
- DOI: http://doi.org/10.4110/in.2012.12.6.269
Abstract
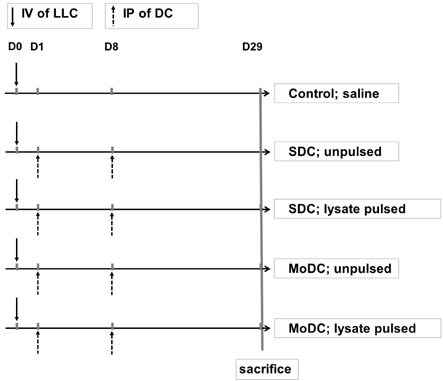
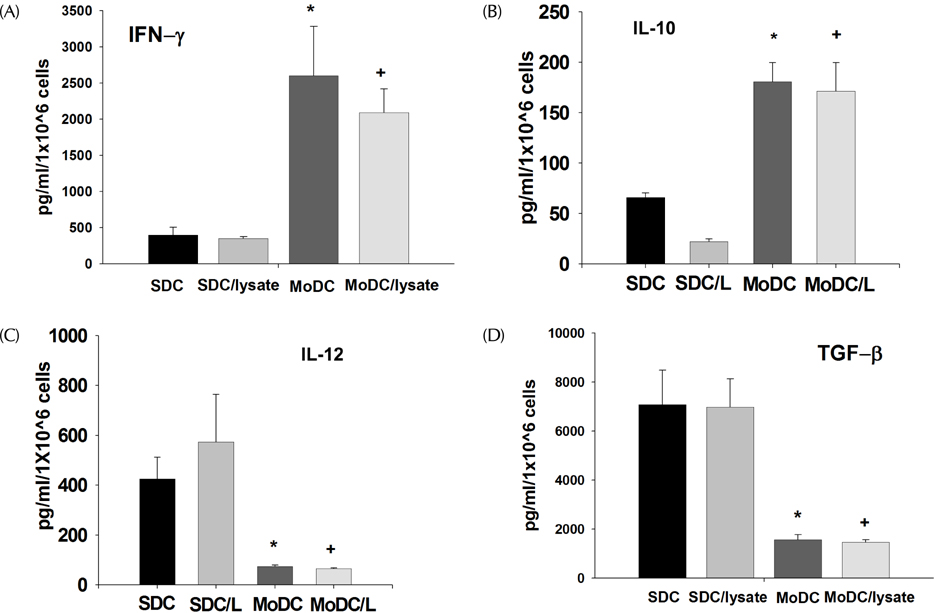
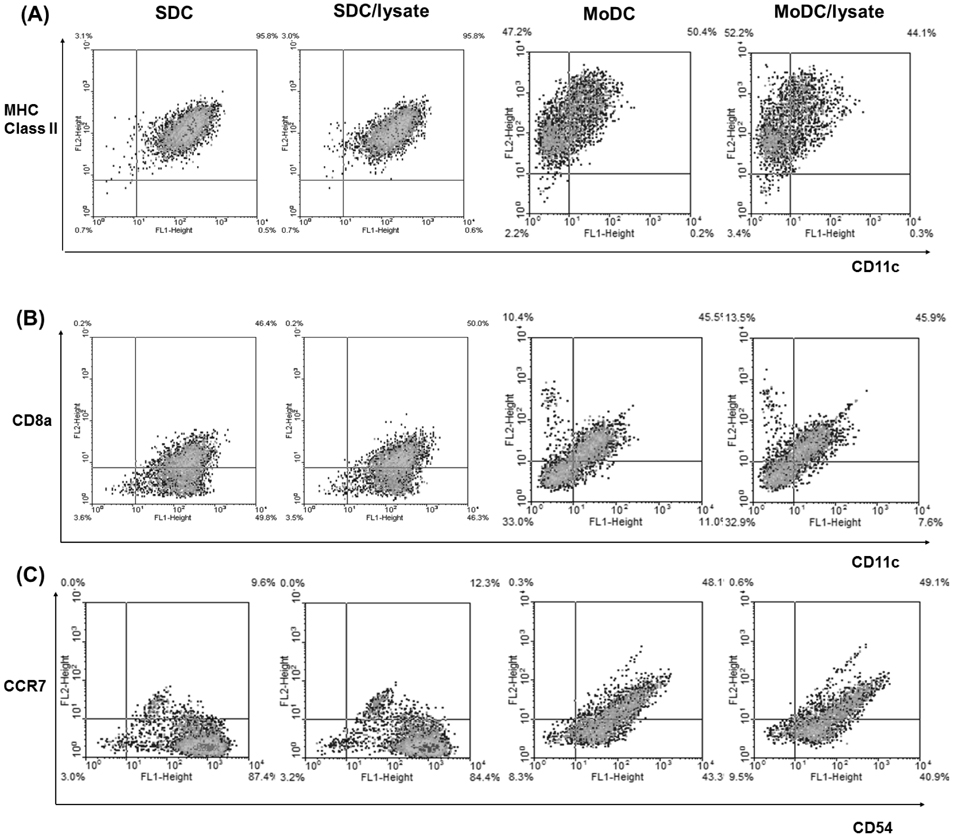
- The anti-tumor effect of monocyte-derived DC (MoDC) vaccine was studied in lung cancer model with feasible but weak Ag-specific immune response and incomplete blocking of tumor growth. To overcome this limitation, the hematopoietic stem cell-derived DC (SDC) was cultured and the anti-tumor effect of MoDC & SDC was compared in mouse lung cancer minimal residual model (MRD). Therapeutic DCs were cultured from either CD34+ hematopoietic stem cells with GM-CSF, SCF and IL-4 for 14 days (SDC) or monocytes with GM-CSF and IL-4 for 7 days (MoDC). DCs were injected twice by one week interval into the peritoneum of mice that are inoculated with Lewis Lung Carcinoma cells (LLC) one day before the DC injection. Anti-tumor responses and the immune modulation were observed 3 weeks after the final DC injection. CD11c expression, IL-12 and TGF-beta secretion were higher in SDC but CCR7 expression, IFN-gamma and IL-10 secretion were higher in MoDC. The proportion of CD11c+CD8a+ cells was similar in both DC cultures. Although both DC reduced the tumor burden, histological anti-tumor effect and the frequencies of IFN-gamma secreting CD8+ T cells were higher in SDC treated group than in MoDC. Conclusively, although both MoDC and SDC can induce the anti-tumor immunity, SDC may be better module as anti-tumor vaccine than MoDC in mouse lung cancer.
Keyword
MeSH Terms
-
Animals
Carcinoma, Lewis Lung
Dendritic Cells
Granulocyte-Macrophage Colony-Stimulating Factor
Hematopoietic Stem Cells
Interleukin-10
Interleukin-12
Interleukin-4
Lung
Lung Neoplasms
Mice
Monocytes
Peritoneum
T-Lymphocytes
Transforming Growth Factor beta
Tumor Burden
Granulocyte-Macrophage Colony-Stimulating Factor
Interleukin-10
Interleukin-12
Interleukin-4
Transforming Growth Factor beta
Figure
Cited by 2 articles
-
Tumor Cell Clone Expressing the Membrane-bound Form of IL-12p35 Subunit Stimulates Antitumor Immune Responses Dominated by CD8+ T Cells
Hoyong Lim, Seon Ah Do, Sang Min Park, Young Sang Kim
Immune Netw. 2013;13(2):63-69. doi: 10.4110/in.2013.13.2.63.Distinct features of dendritic cell-based immunotherapy as cancer vaccines
Chaelin Lee, Myungmi Lee, Inmoo Rhee
Clin Exp Vaccine Res. 2018;7(1):16-23. doi: 10.7774/cevr.2018.7.1.16.
Reference
-
1. Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991. 9:271–296.
Article2. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998. 392:245–252.
Article3. Paglia P, Chiodoni C, Rodolfo M, Colombo MP. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J Exp Med. 1996. 183:317–322.
Article4. Palucka K, Banchereau J. Dendritic cells: a link between innate and adaptive immunity. J Clin Immunol. 1999. 19:12–25.5. Paczesny S, Banchereau J, Wittkowski KM, Saracino G, Fay J, Palucka AK. Expansion of melanoma-specific cytolytic CD8+ T cell precursors in patients with metastatic melanoma vaccinated with CD34+ progenitor-derived dendritic cells. J Exp Med. 2004. 199:1503–1511.
Article6. Kim JH, Lee Y, Bae YS, Kim WS, Kim K, Im HY, Kang WK, Park K, Choi HY, Lee HM, Baek SY, Lee H, Doh H, Kim BM, Kim CY, Jeon C, Jung CW. Phase I/II study of immunotherapy using autologous tumor lysate-pulsed dendritic cells in patients with metastatic renal cell carcinoma. Clin Immunol. 2007. 125:257–267.
Article7. Baek S, Kim CS, Kim SB, Kim YM, Kwon SW, Kim Y, Kim H, Lee H. Combination therapy of renal cell carcinoma or breast cancer patients with dendritic cell vaccine and IL-2: results from a phase I/II trial. J Transl Med. 2011. 9:178.
Article8. Ragde H, Cavanagh WA, Tjoa BA. Dendritic cell based vaccines: progress in immunotherapy studies for prostate cancer. J Urol. 2004. 172:2532–2538.
Article9. Iwashita Y, Tahara K, Goto S, Sasaki A, Kai S, Seike M, Chen CL, Kawano K, Kitano S. A phase I study of autologous dendritic cell-based immunotherapy for patients with unresectable primary liver cancer. Cancer Immunol Immunother. 2003. 52:155–161.
Article10. Hirschowitz EA, Foody T, Kryscio R, Dickson L, Sturgill J, Yannelli J. Autologous dendritic cell vaccines for non-small-cell lung cancer. J Clin Oncol. 2004. 22:2808–2815.
Article11. Hege KM, Carbone DP. Lung cancer vaccines and gene therapy. Lung Cancer. 2003. 41 Suppl 1:S103–S113.
Article12. Fong L, Hou Y, Rivas A, Benike C, Yuen A, Fisher GA, Davis MM, Engleman EG. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci U S A. 2001. 98:8809–8814.
Article13. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005. 55:74–108.
Article14. Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med. 2004. 350:379–392.
Article15. Lee SJ, Kim MJ, In SH, Baek S, Lee H. Immunocell Therapy for Lung Cancer: Dendritic Cell Based Adjuvant Therapy in Mouse Lung Cancer Model. Immune Netw. 2005. 5:36–44.
Article16. Ward KA, Stewart LA, Schwarer AP. CD34+-derived CD11c+ + + BDCA-1+ + CD123+ + DC: expansion of a phenotypically undescribed myeloid DC1 population for use in adoptive immunotherapy. Cytotherapy. 2006. 8:130–140.
Article17. Encabo A, Solves P, Mateu E, Sepúlveda P, Carbonell-Uberos F, Miñana MD. Selective generation of different dendritic cell precursors from CD34+ cells by interleukin-6 and interleukin-3. Stem Cells. 2004. 22:725–740.
Article18. Guo G, Chen S, Zhang J, Luo L, Yu J, Dong H, Xu H, Su Z, Wu L. Antitumor activity of a fusion of esophageal carcinoma cells with dendritic cells derived from cord blood. Vaccine. 2005. 23:5225–5230.
Article19. Xu RL, Tang Y, Ogburn PL, Malinowski K, Madajewicz S, Santiago-Schwarz F, Fan Q. Implication of delayed TNF-alpha exposure on dendritic cell maturation and expansion from cryopreserved cord blood CD34+ hematopoietic progenitors. J Immunol Methods. 2004. 293:169–182.
Article20. Baleeiro RB, Anselmo LB, Soares FA, Pinto CA, Ramos O, Gross JL, Haddad F, Younes RN, Tomiyoshi MY, Bergami-Santos PC, Barbuto JA. High frequency of immature dendritic cells and altered in situ production of interleukin-4 and tumor necrosis factor-alpha in lung cancer. Cancer Immunol Immunother. 2008. 57:1335–1345.
Article21. Hirschowitz EA, Foody T, Hidalgo GE, Yannelli JR. Immunization of NSCLC patients with antigen-pulsed immature autologous dendritic cells. Lung Cancer. 2007. 57:365–372.
Article22. Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000. 1:199–205.
Article23. Martín P, del Hoyo GM, Anjuère F, Ruiz SR, Arias CF, Marín AR, Ardavín C. Concept of lymphoid versus myeloid dendritic cell lineages revisited: both CD8alpha(-) and CD8alpha(+) dendritic cells are generated from CD4(low) lymphoid-committed precursors. Blood. 2000. 96:2511–2519.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-cancer Effect of Hematopoietic Stem Cell-derived Allogeneic-DC Vaccine in Melanoma Metastasis Model
- The Clinical Impact of the Dendritic Cell-based Cancer Vaccine: the Role in the Inflammatory Tumor Micro-environment
- Induction of Dendritic Cell and Cytokine Gene Expression by In situ Delivery of Flt3 Ligand Plasmid
- Immunotherapy using Dendritic Cells against Acute Myelogenous Leukemia
- Immunocell Therapy for Lung Cancer: Dendritic Cell Based Adjuvant Therapy in Mouse Lung Cancer Model