Korean J Physiol Pharmacol.
2016 Jan;20(1):119-127. 10.4196/kjpp.2016.20.1.119.
Comparison of electrophysiological effects of calcium channel blockers on cardiac repolarization
- Affiliations
-
- 1Next-generation Pharmaceutical Research Center, Korea Institute of Toxicology, Daejeon 34114, Korea. idkks@kitox.re.kr
- 2Department of Physiology, Seoul National University College of Medicine, Seoul 03080, Korea. sjoonkim@snu.ac.kr
- 3Human and Environmental Toxicology Program, University of Science and Technology, Daejeon 34113, Korea.
- KMID: 2150481
- DOI: http://doi.org/10.4196/kjpp.2016.20.1.119
Abstract
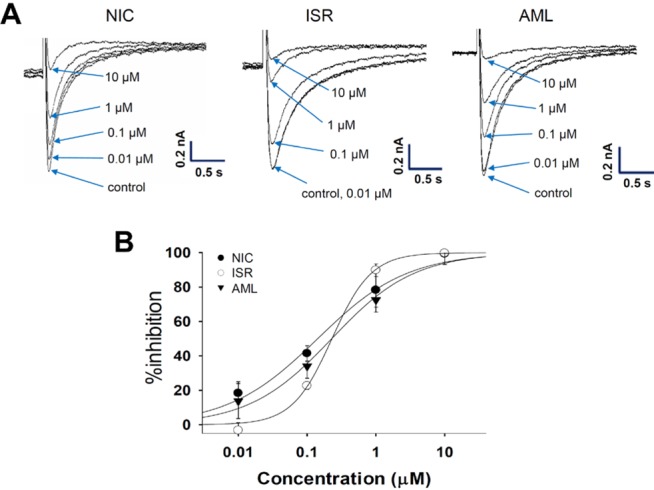
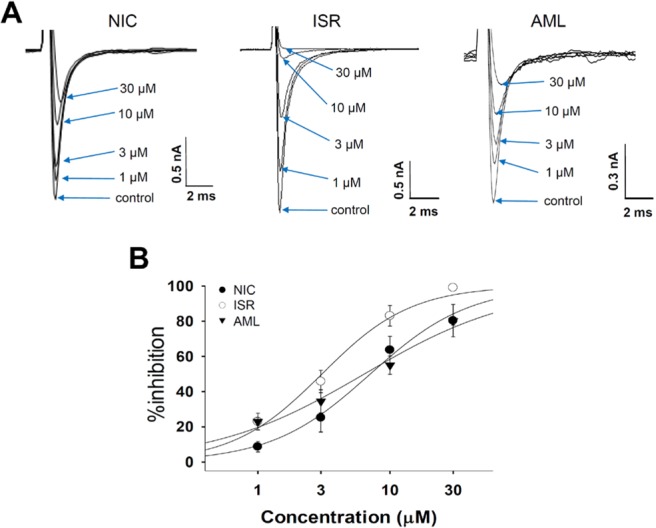
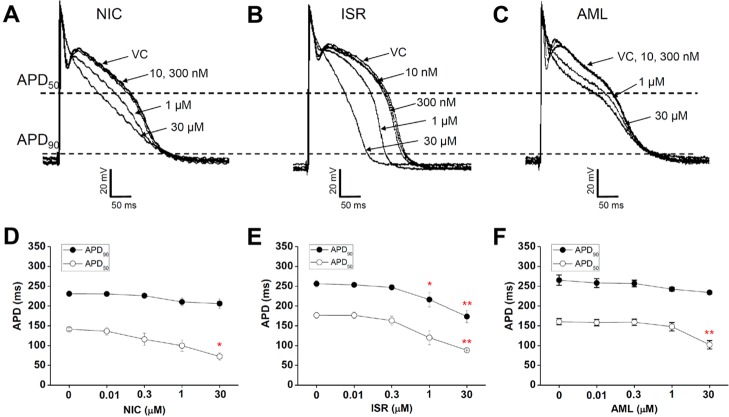
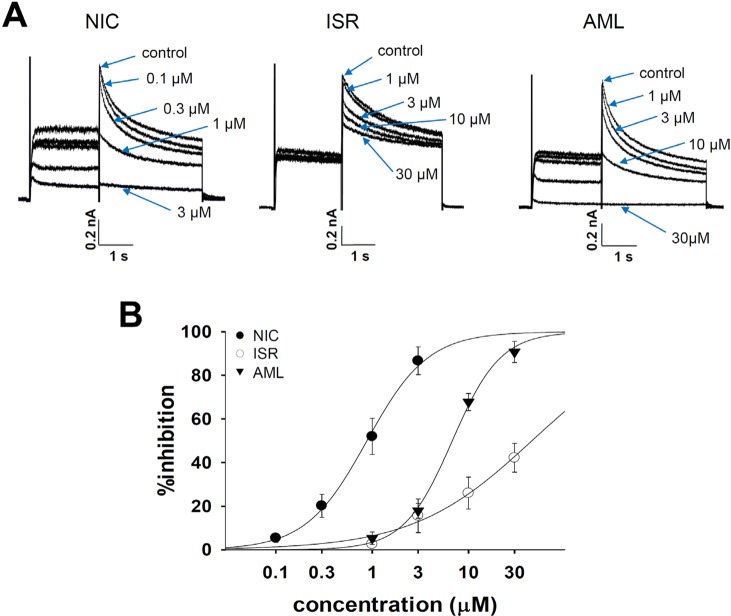
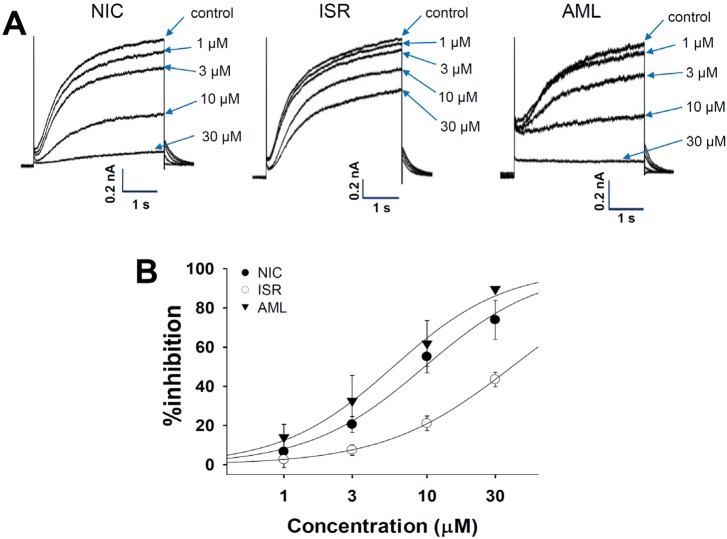
- Dihydropyridine (DHP) calcium channel blockers (CCBs) have been widely used to treat of several cardiovascular diseases. An excessive shortening of action potential duration (APD) due to the reduction of Ca2+ channel current (I(Ca)) might increase the risk of arrhythmia. In this study we investigated the electrophysiological effects of nicardipine (NIC), isradipine (ISR), and amlodipine (AML) on the cardiac APD in rabbit Purkinje fibers, voltage-gated K+ channel currents (I(Kr), I(Ks)) and voltage-gated Na+ channel current (I(Na)). The concentration-dependent inhibition of Ca2+ channel currents (I(Ca)) was examined in rat cardiomyocytes; these CCBs have similar potency on I(Ca) channel blocking with IC50 (the half-maximum inhibiting concentration) values of 0.142, 0.229, and 0.227 nM on NIC, ISR, and AML, respectively. However, ISR shortened both APD50 and APD90 already at 1 microM whereas NIC and AML shortened APD50 but not APD90 up to 30 microM. According to ion channel studies, NIC and AML concentration-dependently inhibited I(Kr) and I(Ks) while ISR had only partial inhibitory effects (<50% at 30 microM). Inhibition of I(Na) was similarly observed in the three CCBs. Since the I(Kr) and I(Ks) mainly contribute to cardiac repolarization, their inhibition by NIC and AML could compensate for the AP shortening effects due to the block of I(Ca).
Keyword
MeSH Terms
-
Action Potentials
Amlodipine
Animals
Antihypertensive Agents
Arrhythmias, Cardiac
Calcium Channel Blockers*
Calcium Channels*
Calcium*
Cardiovascular Diseases
Inhibitory Concentration 50
Ion Channels
Isradipine
Myocytes, Cardiac
Nicardipine
Purkinje Fibers
Rats
Amlodipine
Antihypertensive Agents
Calcium
Calcium Channel Blockers
Calcium Channels
Ion Channels
Isradipine
Nicardipine
Figure
Cited by 1 articles
-
Inhibition of voltage-dependent K+ current in rabbit coronary arterial smooth muscle cells by the class Ic antiarrhythmic drug propafenone
Jin Ryeol An, Hongliang Li, Mi Seon Seo, Won Sun Park
Korean J Physiol Pharmacol. 2018;22(5):597-605. doi: 10.4196/kjpp.2018.22.5.597.
Reference
-
1. Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J. ASCOT Investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005; 366:895–906. PMID: 16154016.
Article2. Siragy HM. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitors or calcium channel blocker vs diuretic. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Curr Hypertens Rep. 2003; 5:293–294. PMID: 12844462.3. Black HR, Elliott WJ, Grandits G, Grambsch P, Lucente T, White WB, Neaton JD, Grimm RH Jr, Hansson L, Lacourciere Y, Muller J, Sleight P, Weber MA, Williams G, Wittes J, Zanchetti A, Anders RJ. CONVINCE Research Group. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003; 289:2073–2082. PMID: 12709465.4. Pepine CJ, Handberg EM, Cooper-DeHoff RM, Marks RG, Kowey P, Messerli FH, Mancia G, Cangiano JL, Garcia-Barreto D, Keltai M, Erdine S, Bristol HA, Kolb HR, Bakris GL, Cohen JD, Parmley WW. INVEST Investigators. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003; 290:2805–2816. PMID: 14657064.
Article5. Burnier M, Pruijm M, Wuerzner G. Treatment of essential hypertension with calcium channel blockers: what is the place of lercanidipine? Expert Opin Drug Metab Toxicol. 2009; 5:981–987. PMID: 19619074.
Article6. Hirano Y, Hiraoka M. Electrophysiology of cardiac ion channels. Nihon Rinsho. 1996; 54:2050–2055. PMID: 8810776.7. DeWitt CR, Waksman JC. Pharmacology, pathophysiology and management of calcium channel blocker and beta-blocker toxicity. Toxicol Rev. 2004; 23:223–238. PMID: 15898828.8. Priest BT, Bell IM, Garcia ML. Role of hERG potassium channel assays in drug development. Channels (Austin). 2008; 2:87–93. PMID: 18849661.
Article9. Barhanin J, Attali B, Lazdunski M. IKs, a slow and intriguing cardiac K+ channel and its associated long QT diseases. Trends Cardiovasc Med. 1998; 8:207–214. PMID: 14987566.10. Haverkamp W, Breithardt G, Camm AJ, Janse MJ, Rosen MR, Antzelevitch C, Escande D, Franz M, Malik M, Moss A, Shah R. The potential for QT prolongation and proarrhythmia by nonantiarrhythmic drugs: clinical and regulatory implications. Report on a policy conference of the European Society of Cardiology. Eur Heart J. 2000; 21:1216–1231. PMID: 10924311.
Article11. Cubeddu LX. QT prolongation and fatal arrhythmias: a review of clinical implications and effects of drugs. Am J Ther. 2003; 10:452–457. PMID: 14624285.
Article12. Gaita F, Giustetto C, Bianchi F, Wolpert C, Schimpf R, Riccardi R, Grossi S, Richiardi E, Borggrefe M. Short QT Syndrome: a familial cause of sudden death. Circulation. 2003; 108:965–970. PMID: 12925462.13. Giustetto C, Di Monte F, Wolpert C, Borggrefe M, Schimpf R, Sbragia P, Leone G, Maury P, Anttonen O, Haissaguerre M, Gaita F. Short QT syndrome: clinical findings and diagnostic-therapeutic implications. Eur Heart J. 2006; 27:2440–2447. PMID: 16926178.
Article14. Extramiana F, Antzelevitch C. Amplified transmural dispersion of repolarization as the basis for arrhythmogenesis in a canine ventricular-wedge model of short-QT syndrome. Circulation. 2004; 110:3661–3666. PMID: 15569843.
Article15. Hondeghem LM. Thorough QT/QTc not so thorough: removes torsadogenic predictors from the T-wave, incriminates safe drugs, and misses profibrillatory drugs. J Cardiovasc Electrophysiol. 2006; 17:337–340. PMID: 16643415.
Article16. Garberoglio L, Giustetto C, Wolpert C, Gaita F. Is acquired short QT due to digitalis intoxication responsible for malignant ventricular arrhythmias? J Electrocardiol. 2007; 40:43–46. PMID: 16950330.17. Lu HR, Vlaminckx E, Hermans AN, Rohrbacher J, Van Ammel K, Towart R, Pugsley M, Gallacher DJ. Predicting drug-induced changes in QT interval and arrhythmias: QT-shortening drugs point to gaps in the ICHS7B Guidelines. Br J Pharmacol. 2008; 154:1427–1438. PMID: 18493243.
Article18. Aubert M, Osterwalder R, Wagner B, Parrilla I, Cavero I, Doessegger L, Ertel EA. Evaluation of the rabbit Purkinje fibre assay as an in vitro tool for assessing the risk of drug-induced torsades de pointes in humans. Drug Saf. 2006; 29:237–254. PMID: 16524323.
Article19. Food and Drug Administration, HHS. International Conference on Harmonisation; guidance on S7B Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization (QT Interval Prolongation) by Human Pharmaceuticals; availability. Notice. Fed Regist. 2005; 70:61133–61134. PMID: 16237859.20. D'Alonzo AJ, Hess TA, Darbenzio RB, Sewter JC, Conder ML, McCullough JR. Effects of cromakalim or pinacidil on pacing- and ischemia-induced ventricular fibrillation in the anesthetized pig. Basic Res Cardiol. 1994; 89:163–176. PMID: 8074640.21. D'Alonzo AJ, Zhu JL, Darbenzio RB, Dorso CR, Grover GJ. Proarrhythmic effects of pinacidil are partially mediated through enhancement of catecholamine release in isolated perfused guineapig hearts. J Mol Cell Cardiol. 1998; 30:415–423. PMID: 9515018.22. Extramiana F, Antzelevitch C. Amplified transmural dispersion of repolarization as the basis for arrhythmogenesis in a canine ventricular-wedge model of short-QT syndrome. Circulation. 2004; 110:3661–3666. PMID: 15569843.
Article23. Hondeghem LM. Thorough QT/QTc not so thorough: removes torsadogenic predictors from the T-wave, incriminates safe drugs, and misses profibrillatory drugs. J Cardiovasc Electrophysiol. 2006; 17:337–340. PMID: 16643415.
Article24. Garberoglio L, Giustetto C, Wolpert C, Gaita F. Is acquired short QT due to digitalis intoxication responsible for malignant ventricular arrhythmias? J Electrocardiol. 2007; 40:43–46. PMID: 16950330.25. Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004; 350:1013–1022. PMID: 14999113.
Article26. Hondeghem LM, Carlsson L, Duker G. Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation. 2001; 103:2004–2013. PMID: 11306531.
Article27. Hondeghem LM. QT and TdP. QT: an unreliable predictor of proarrhythmia. Acta Cardiol. 2008; 63:1–7. PMID: 18372573.28. Milberg P, Eckardt L, Bruns HJ, Biertz J, Ramtin S, Reinsch N, Fleischer D, Kirchhof P, Fabritz L, Breithardt G, Haverkamp W. Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointes. J Pharmacol Exp Ther. 2002; 303:218–225. PMID: 12235254.
Article29. Lu HR, Vlaminckx E, Van Ammel K, De Clerck F. Drug-induced long QT in isolated rabbit Purkinje fibers: importance of action potential duration, triangulation and early afterdepolarizations. Eur J Pharmacol. 2002; 452:183–192. PMID: 12354568.
Article30. Champeroux P, Viaud K, El Amrani AI, Fowler JS, Martel E, Le Guennec JY, Richard S. Prediction of the risk of Torsade de Pointes using the model of isolated canine Purkinje fibres. Br J Pharmacol. 2005; 144:376–385. PMID: 15655517.
Article31. Guo D, Zhao X, Wu Y, Liu T, Kowey PR, Yan GX. L-type calcium current reactivation contributes to arrhythmogenesis associated with action potential triangulation. J Cardiovasc Electrophysiol. 2007; 18:196–203. PMID: 17212595.
Article32. Packer M, O'Connor CM, Ghali JK, Pressler ML, Carson PE, Belkin RN, Miller AB, Neuberg GW, Frid D, Wertheimer JH, Cropp AB, DeMets DL. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. Prospective Randomized Amlodipine Survival Evaluation Study Group. N Engl J Med. 1996; 335:1107–1114. PMID: 8813041.33. O'Connor CM, Carson PE, Miller AB, Pressler ML, Belkin RN, Neuberg GW, Frid DJ, Cropp AB, Anderson S, Wertheimer JH, DeMets DL. Effect of amlodipine on mode of death among patients with advanced heart failure in the PRAISE trial. Prospective Randomized Amlodipine Survival Evaluation. Am J Cardiol. 1998; 82:881–887. PMID: 9781971.34. Udelson JE, DeAbate CA, Berk M, Neuberg G, Packer M, Vijay NK, Gorwitt J, Smith WB, Kukin ML, LeJemtel T, Levine TB, Konstam MA. Effects of amlodipine on exercise tolerance, quality of life, and left ventricular function in patients with heart failure from left ventricular systolic dysfunction. Am Heart J. 2000; 139:503–510. PMID: 10689266.
Article35. Littler WA, Sheridan DJ. Placebo controlled trial of felodipine in patients with mild to moderate heart failure. UK Study Group. Br Heart J. 1995; 73:428–433. PMID: 7786657.
Article36. Li K, Zhang X, Yuan YS, Zhao FL. A high-performance liquid chromatographic method for the determination of nicardipine in plasma and its application to pharmacokinetics in humans. Biomed Chromatogr. 1998; 12:326–329. PMID: 9861491.
Article37. Christensen HR, Antonsen K, Simonsen K, Lindekaer A, Bonde J, Angelo HR, Kampmann JP. Bioavailability and pharmacokinetics of isradipine after oral and intravenous administration: half-life shorter than expected? Pharmacol Toxicol. 2000; 86:178–182. PMID: 10815751.
Article38. Elliott HL, Meredith PA, Reid JL, Faulkner JK. A comparison of the disposition of single oral doses of amlodipine in young and elderly subjects. J Cardiovasc Pharmacol. 1988; 12(Suppl 7):S64–S66.
Article39. van Zwieten PA, Pfaffendorf M. Similarities and differences between calcium antagonists: pharmacological aspects. J Hypertens Suppl. 1993; 11:S3–S11.
Article40. Schoffstall JM, Spivey WH, Gambone LM, Shaw RP, Sit SP. Effects of calcium channel blocker overdose-induced toxicity in the conscious dog. Ann Emerg Med. 1991; 20:1104–1108. PMID: 1928882.
Article41. Lee SR, Noh SJ, Pronto JR, Jeong YJ, Kim HK, Song IS, Xu Z, Kwon HY, Kang SC, Sohn EH, Ko KS, Rhee BD, Kim N, Han J. The critical roles of zinc: beyond impact on myocardial signaling. Korean J Physiol Pharmacol. 2015; 19:389–399. PMID: 26330751.
Article42. Yun J, Bae H, Choi SE, Kim JH, Choi YW, Lim I, Lee CS, Lee MW, Ko JH, Seo SJ, Bang H. Taxifolin Glycoside Blocks Human ethera-go-go Related Gene K+ Channels. Korean J Physiol Pharmacol. 2013; 17:37–42. PMID: 23440017.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Calcium Channel Blockers on Cardiopulmonary Hemodynamics in Dogs
- A case of gingival hyperplasia caused by amlodipine
- The Effect of Oral Calcium Channel Blockers on the Ocular Blood Flow
- Effects of alpha-, beta-adrenergic, and calcium channel blockers on renin- angiotensin system in perfused rat heart
- A case of gingival hyperplasia caused by felodipine