Ann Rehabil Med.
2015 Oct;39(5):667-675. 10.5535/arm.2015.39.5.667.
The Effect of Pulsed Radiofrequency Applied to the Peripheral Nerve in Chronic Constriction Injury Rat Model
- Affiliations
-
- 1Department of Physical and Rehabilitation Medicine, Research Institute of Medical Sciences, Chonnam National University Medical School & Hospital, Gwangju, Korea. drchoiis@naver.com
- 2Department of Pathology, Research Institute of Medical Sciences, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2148197
- DOI: http://doi.org/10.5535/arm.2015.39.5.667
Abstract
OBJECTIVE
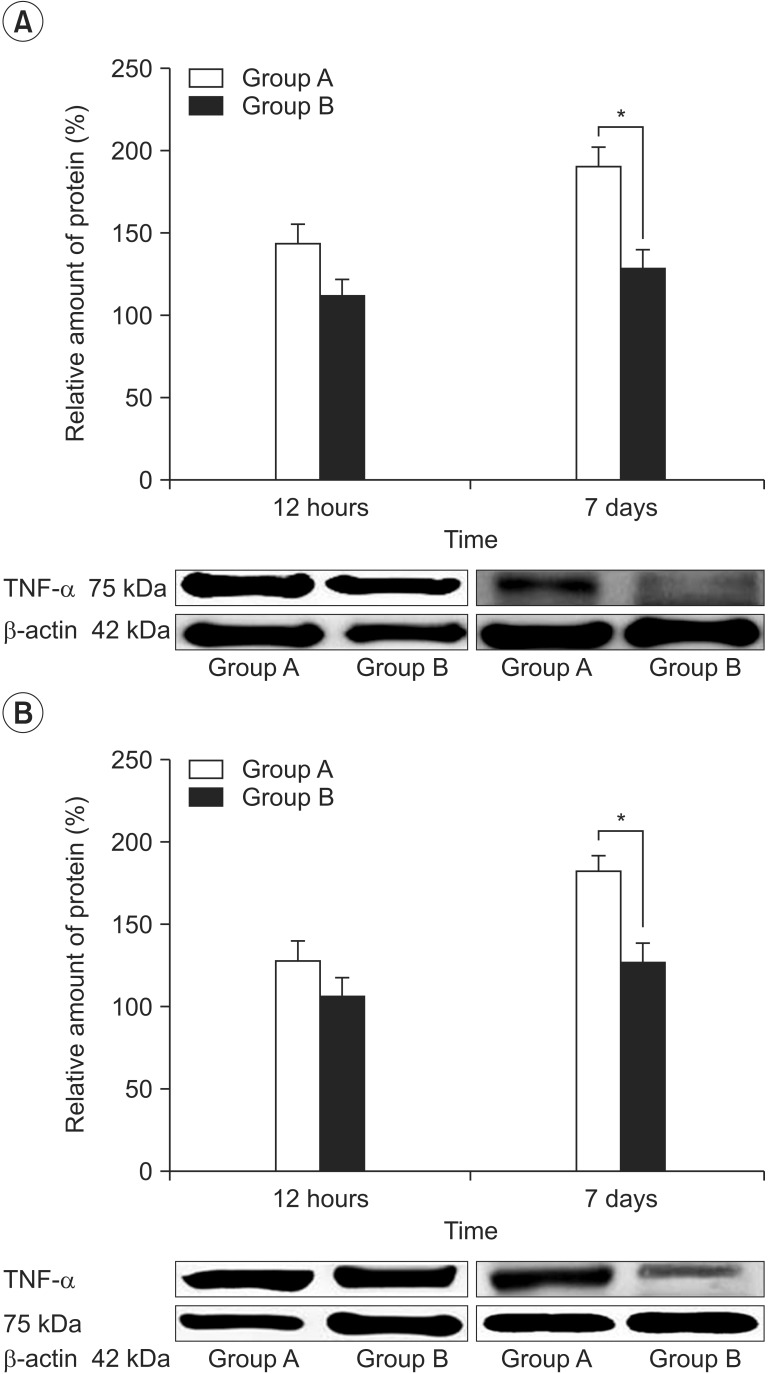
To investigate the effect of pulsed radiofrequency (PRF) applied proximal to the injured peripheral nerve on the expression of tumor necrosis factor-alpha (TNF-alpha) in a neuropathic pain rat model.
METHODS
Nineteen male Sprague-Dawley rats were used in the study. All rats underwent chronic constriction injury (CCI) procedure. After 7 days of CCI, withdrawal frequency of affected hind paw to mechanical stimuli and withdrawal latency of affected hind paw to heat stimulus were measured. They were randomly divided into two groups: group A, CCI group (n=9) and group B, CCI treated with PRF group (n=10). Rats of group B underwent PRF procedure on the sciatic nerve. Withdrawal frequency and withdrawal latency were measured at 12 hours, and 7 days after PRF. Immunohistochemistry and Western blot analysis were performed using a TNF-alpha antibody.
RESULTS
Before PRF, withdrawal frequency and withdrawal latency were not different in both groups. After PRF, withdrawal frequency decreased and withdrawal latency prolonged over time in group B. There was significant interaction between time and group for each withdrawal frequency and withdrawal latency. Group B showed decreased TNF-alpha immunoreactivity of the spinal cord and sciatic nerve at 7 days.
CONCLUSION
PRF applied proximal to the peripheral nerve injury is potentially helpful for the reduction of neuropathic pain by neuromodulation of inflammatory markers.
MeSH Terms
Figure
Reference
-
1. The IASP Taxonomy Working Group. Classification of chronic pain. 2nd rev. ed. Seattle: IASP Press;2012.2. Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007; 132:237–251. PMID: 17920770.
Article3. Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology. 2007; 68:1178–1182. PMID: 17420400.
Article4. van Wijk RM, Geurts JW, Wynne HJ. Long-lasting analgesic effect of radiofrequency treatment of the lumbosacral dorsal root ganglion. J Neurosurg. 2001; 94(2 Suppl):227–231. PMID: 11302625.
Article5. Pagura JR. Percutaneous radiofrequency spinal rhizotomy. Appl Neurophysiol. 1983; 46:138–146. PMID: 6670863.
Article6. Vatansever D, Tekin I, Tuglu I, Erbuyun K, Ok G. A comparison of the neuroablative effects of conventional and pulsed radiofrequency techniques. Clin J Pain. 2008; 24:717–724. PMID: 18806537.
Article7. Heavner JE, Boswell MV, Racz GB. A comparison of pulsed radiofrequency and continuous radiofrequency on thermocoagulation of egg white in vitro. Pain Physician. 2006; 9:135–137. PMID: 16703974.8. Choi GS, Ahn SH, Cho YW, Lee DK. Short-term effects of pulsed radiofrequency on chronic refractory cervical radicular pain. Ann Rehabil Med. 2011; 35:826–832. PMID: 22506211.
Article9. Higuchi Y, Nashold BS Jr, Sluijter M, Cosman E, Pearlstein RD. Exposure of the dorsal root ganglion in rats to pulsed radiofrequency currents activates dorsal horn lamina I and II neurons. Neurosurgery. 2002; 50:850–856. PMID: 11904038.
Article10. Xu JT, Xin WJ, Zang Y, Wu CY, Liu XG. The role of tumor necrosis factor-alpha in the neuropathic pain induced by Lumbar 5 ventral root transection in rat. Pain. 2006; 123:306–321. PMID: 16675114.
Article11. Sommer C, Schmidt C, George A. Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp Neurol. 1998; 151:138–142. PMID: 9582261.
Article12. Leung L, Cahill CM. TNF-alpha and neuropathic pain: a review. J Neuroinflammation. 2010; 7:27. PMID: 20398373.13. Jancalek R, Dubovt P, Svizenska I, Klusakova I. Bilateral changes of TNF-alpha and IL-10 protein in the lumbar and cervical dorsal root ganglia following a unilateral chronic constriction injury of the sciatic nerve. J Neuroinflammation. 2010; 7:11. PMID: 20146792.
Article14. Fowler IM, Tucker AA, Mendez RJ. Treatment of meralgia paresthetica with ultrasound-guided pulsed radiofrequency ablation of the lateral femoral cutaneous nerve. Pain Pract. 2012; 12:394–398. PMID: 22151457.
Article15. Wu YT, Ho CW, Chen YL, Li TY, Lee KC, Chen LC. Ultrasound-guided pulsed radiofrequency stimulation of the suprascapular nerve for adhesive capsulitis: a prospective, randomized, controlled trial. Anesth Analg. 2014; 119:686–692. PMID: 25010824.16. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988; 33:87–107. PMID: 2837713.
Article17. Ko HY, Shin YB, Lee SH, Moohn HN, Kwon DR, Ahn YH. Effect of radiofrequency lesioning on peripheral nerve conductivity in relation to distance between lesioning electrode and target tissue in rats. J Korean Acad Rehabil Med. 2004; 28:449–453.18. Park CH, Lee YW, Kim YC, Moon JH, Choi JB. Treatment experience of pulsed radiofrequency under ultrasound guided to the trapezius muscle at myofascial pain syndrome: a case report. Korean J Pain. 2012; 25:52–54. PMID: 22259718.
Article19. Podhajsky RJ, Sekiguchi Y, Kikuchi S, Myers RR. The histologic effects of pulsed and continuous radiofrequency lesions at 42 degrees C to rat dorsal root ganglion and sciatic nerve. Spine (Phila Pa 1976). 2005; 30:1008–1013. PMID: 15864151.20. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63. PMID: 7990513.
Article21. Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988; 32:77–88. PMID: 3340425.
Article22. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009; 32:1–32. PMID: 19400724.
Article23. Maves TJ, Pechman PS, Gebhart GF, Meller ST. Possible chemical contribution from chromic gut sutures produces disorders of pain sensation like those seen in man. Pain. 1993; 54:57–69. PMID: 8378104.
Article24. Sommer C, Galbraith JA, Heckman HM, Myers RR. Pathology of experimental compression neuropathy producing hyperesthesia. J Neuropathol Exp Neurol. 1993; 52:223–233. PMID: 8492140.
Article25. Clatworthy AL, Illich PA, Castro GA, Walters ET. Role of peri-axonal inflammation in the development of thermal hyperalgesia and guarding behavior in a rat model of neuropathic pain. Neurosci Lett. 1995; 184:5–8. PMID: 7739805.
Article26. Wagner R, Myers RR. Schwann cells produce tumor necrosis factor alpha: expression in injured and non-injured nerves. Neuroscience. 1996; 73:625–629. PMID: 8809782.
Article27. Wagner R, Myers RR. Endoneurial injection of TNF-alpha produces neuropathic pain behaviors. Neuroreport. 1996; 7:2897–2901. PMID: 9116205.28. Kukkar A, Singh N, Jaggi AS. Neuropathic pain-attenuating potential of aliskiren in chronic constriction injury model in rats. J Renin Angiotensin Aldosterone Syst. 2013; 14:116–123. PMID: 23087256.
Article29. Wagner R, Janjigian M, Myers RR. Anti-inflammatory interleukin-10 therapy in CCI neuropathy decreases thermal hyperalgesia, macrophage recruitment, and endoneurial TNF-alpha expression. Pain. 1998; 74:35–42. PMID: 9514558.30. Cui JG, Holmin S, Mathiesen T, Meyerson BA, Linderoth B. Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain. 2000; 88:239–248. PMID: 11068111.
Article31. Shubayev VI, Myers RR. Upregulation and interaction of TNF alpha and gelatinases A and B in painful peripheral nerve injury. Brain Res. 2000; 855:83–89. PMID: 10650133.32. Pathak NN, Balaganur V, Lingaraju MC, More AS, Kant V, Kumar D, et al. Antihyperalgesic and anti-inflammatory effects of atorvastatin in chronic constriction injury-induced neuropathic pain in rats. Inflammation. 2013; 36:1468–1478. PMID: 23872719.
Article33. Erdine S, Yucel A, Cimen A, Aydin S, Sav A, Bilir A. Effects of pulsed versus conventional radiofrequency current on rabbit dorsal root ganglion morphology. Eur J Pain. 2005; 9:251–256. PMID: 15862474.
Article34. Tanaka N, Yamaga M, Tateyama S, Uno T, Tsuneyoshi I, Takasaki M. The effect of pulsed radiofrequency current on mechanical allodynia induced with resiniferatoxin in rats. Anesth Analg. 2010; 111:784–790. PMID: 20601454.
Article35. Cosman ER Jr, Cosman ER Sr. Electric and thermal field effects in tissue around radiofrequency electrodes. Pain Med. 2005; 6:405–424. PMID: 16336478.
Article36. Abejon D, Reig E. Is pulsed radiofrequency a neuromodulation technique? Neuromodulation. 2003; 6:1–3. PMID: 22150906.37. Munglani R. The longer term effect of pulsed radiofrequency for neuropathic pain. Pain. 1999; 80:437–439. PMID: 10204759.
Article38. Aksu R, Ugur F, Bicer C, Menku A, Guler G, Madenoglu H, et al. The efficiency of pulsed radiofrequency application on L5 and l6 dorsal roots in rabbits developing neuropathic pain. Reg Anesth Pain Med. 2010; 35:11–15. PMID: 20048653.
Article39. Vallejo R, Tilley DM, Williams J, Labak S, Aliaga L, Benyamin RM. Pulsed radiofrequency modulates pain regulatory gene expression along the nociceptive pathway. Pain Physician. 2013; 16:E601–E613. PMID: 24077210.40. Ozsoylar O, Akçali D, Cizmeci P, Babacan A, Cahana A, Bolay H. Percutaneous pulsed radiofrequency reduces mechanical allodynia in a neuropathic pain model. Anesth Analg. 2008; 107:1406–1411. PMID: 18806060.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pulsed radiofrequency lesioning of the median nerve in a patient with bilateral carpal tunnel syndrome: A case report
- Pulsed radiofrequency lesioning for treatment of chronic breast neuropathic pain after breast reduction: A case report
- The effect of pulsed radiofrequency (PRF) for the treatment of supraorbital neuropathic pain: A report of three cases
- Ultrasound-guided pulsed radiofrequency treatment for postherpetic neuralgia of supraorbital nerve: A case report
- Reduction in mechanical allodynia in complex regional pain syndrome patients with ultrasound-guided pulsed radiofrequency treatment of the superficial peroneal nerve